
Gestational gigantomastia complicated by breast infarctive necrosis in the setting of COVID-19 infection: a case report
Introduction
Gestational gigantomastia (GG) is a rare condition of unknown etiology characterized by rapid and significant enlargement of the breast tissue in the peripartum setting (1-3). GG can present with superficial venous dilation and skin ulceration and can be complicated by local infection or sepsis (3-5). We report the first case of infarctive necrosis of the breast in a patient with GG and concurrent COVID-19 infection. We present the following case report in accordance with the CARE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-140/rc).
Case presentation
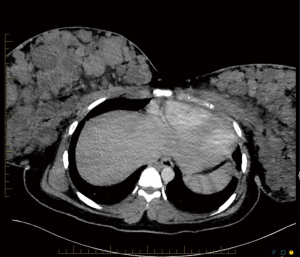
The patient is an 18-year-old black woman, gravida 1 para 1, who delivered at 33 weeks’ gestation after premature prelabor rupture of membranes (PPROM) in the setting of an active COVID-19 infection despite being fully vaccinated for COVID-19 according to vaccination guidelines at the time of presentation. On initial presentation she complained of shortness of breath and chest pressure over the last several days prior to admission. The patient had a body mass index (BMI) of 33.7 at the time of presentation, from a pre-pregnancy BMI of 28.12. On exam, she was afebrile, tachycardic with a heart rate in the 120–150 s, and maintained an appropriate oxygen saturation on room air. Due to her overall mild symptoms of COVID-19, she was not treated with resdemivir or dexamethasone. A CT pulmonary angiogram was negative for pulmonary embolus but noted bilateral nodular densities within both breasts (Figure 1). She delivered a healthy female infant vaginally and her shortness of breath improved. She was discharged home on postpartum day 2.
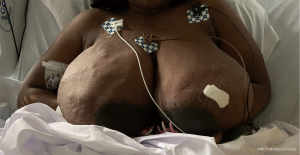
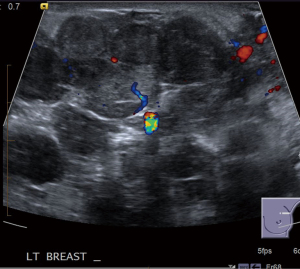
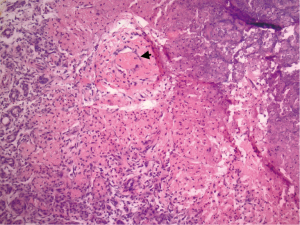
She represented on postpartum day 3 complaining of bilateral breast swelling, erythema, and pain. She was noted to be febrile and tachycardic with leukocytosis, and she was started on empiric broad spectrum antibiotics consisting of vancomycin/piperacillin/tazobactam and subsequently narrowed to treatment with ampicillin/sulbactam due to concern for sepsis. Initially there was concern for retained products of conception based on pelvic imaging. Surgical evacuation of the uterus was therefore performed however did not result in clinical improvement. On clinical exam, her breasts were noted to be significantly enlarged bilaterally, and there were areas of focal erythema and induration (Figure 2). The patient did not raise concern for breast enlargement prior to giving birth. Though the patient’s breast size prior to pregnancy was unknown, the patient had raised concern for back pain due to large breast size at a past wellness visit. No breast masses or lymphadenopathy was appreciated. Bilateral breast ultrasound was performed showing multiple heterogeneous nodules and confluent nodules (Figure 3), and the breast surgical oncology department performed a bedside left breast core needle biopsy targeting a palpable lesion corresponding to one of the hypoechoic nodules seen on diagnostic ultrasound. Pathology showed breast tissue with extensive necrosis most consistent with infarction and associated mild acute inflammation and small vessel thrombus in an area of necrosis (Figure 4). No vasculitis, granulomatous inflammation, or malignancy was identified, and fluid aspirated at the time of the biopsy was not consistent with galactocele. She was started on therapeutic anticoagulation due to concern for septic pelvic thrombophlebitis in the setting of persistent fever after dilation and curettage performed for concern for retained products of conception. Tissue and blood cultures remained negative, antibiotics were discontinued. Anticoagulation was refused by the patient and later discontinued after multidisciplinary discussion. She was ultimately diagnosed with GG and started on bromocriptine 2.5 mg QD for 60 days for lactation suppression. The patient did not attempt breast feeding prior to admission but used a breast pump briefly during admission with partial resolution of chest pain. Over the next 2 to 3 days her breast pain and swelling improved with conservative measures. The patient did not require intensive care for COVID19 infection. She was discharged on hospital day 5 afebrile and without pain.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
GG is an extremely rare condition primarily reported in Asian or African women in their second to fourth decade (1). It can be differentiated from engorgement of the breasts due to lactation based on size of breast enlargement and symptoms (2,3). GG can give rise to various complications including skin ulceration and infection (1,3-5), and the literature reports an approximately 3% mortality rate (4).
To our knowledge, this is the first reported case of breast tissue infarctive necrosis in the setting of GG. This case is also unique in that the patient was COVID-19 positive at the time of diagnosis. Pathology reports of GG biopsies typically show changes consistent with the lactating breast, including stromal hyperplasia, lobular hypertrophy, and tissue fibrosis, but not necrosis or infarction (2,6). Some reviews described a lymphocytic infiltrate to be common, however others report this to be uncommon (3,4,6). Gross skin necrosis has been reported in GG (1). Gross skin necrosis forms large ulcers caused by the rapid growth of breast tissue and angiogenesis within the breast disproportionate to the rate of growth of the skin, rather than the small vessel thrombosis causing the infarctive necrosis in our patient (2). In addition, while fat necrosis is common in the breast, infarctive necrosis is incredibly rare (7). Fat necrosis is caused by hemorrhage in fat tissue, usually due to trauma or surgery, and typically results in gross calcifications and cystic structures in the breast (8). In contrast, the infarctive necrosis seen in our patient demonstrated small vessel thrombosis.
Arterial thrombosis of the breast, as in our patient, is extremely rare with a few cases reported in the setting of systemic thromboembolic disease (9), pseudoaneurysm formation after core needle biopsy (10), and diffuse dermal angiomatosis (7). Our patient had no prior hypercoagulable conditions, but did have symptomatic COVID-19 infection. Up to 79% of COVID-19 cases are complicated by a hypercoagulable state, which is thought to be due to multiple pathways including endothelial surface angiotensin converting enzyme 2 (ACE2) activation and tissue factor activation (11). The most common manifestations are deep vein thrombosis (DVT) and pulmonary embolism (PE), although thrombosis of the liver, spleen, brain, gastrointestinal tract, genitourinary organs, and periphery have been documented (12). It is possible that this patient’s COVID-19 infection led to the infarctive necrosis seen on core biopsy due to a hypercoagulable state. However, as many patients with presumed GG are not biopsied, it is difficult to know the true incidence of infarctive necrosis with GG (10-12).
Treatment for GG relies on medical therapies, but surgical intervention is often necessary. Dopamine agonists, specifically bromocriptine, are the standard medical management for GG (1,2,6). Because multiple masses can be seen on imaging, biopsy is recommended to rule out granulomatous mastitis or other pathologic entities (1). Although GG will resolve spontaneously in some patients, nearly 65% of patients fail medical management and require mastectomy (1,2,6). Our initial approach with this patient included biopsy of a palpable nodule, pain control, medical and nonmedical interventions to stop lactation, prophylactic antibiotics, and anticoagulation (1,2,5). Fortunately, her symptoms abated with conservative management, and she did not require an emergent bilateral mastectomy. Some patients will require an interval bilateral mastectomy after lactation ceases as there is a high possibility of recurrence of GG with subsequent pregnancies (6). Another option is a bilateral breast reduction as a stepping stone to mastectomy particularly if the patient wants to have future pregnancies and breast feed in the future, though conservative management is preferred as breast feeding can be compromised by mammoplasty (13). Partial mammoplasty can compromise lactation depending on the degree of preservation of subareolar parenchymal structure and conservative and medical management remains the preferred first step (13,14). However, GG can have a high recurrence risk and may necessitate mastectomy (2,6).
One might consider further workup and prophylactic treatment of a possible hypercoagulable state, given the small vessel thrombosis seen on our patient’s breast biopsy. While there was limited evidence to support use of thromboprophylaxis in hospitalized patients with COVID-19 infection at the time our patient presented, newer guidelines strongly recommend prophylactic dose anticoagulation in pregnant and non-pregnant infected patients (15,16). There are not recommendations specific for postpartum women (15). Furthermore, a repeat coagulation panel followed by testing for common inherited and acquired hypercoagulability syndromes might have been considered (17,18). However, these would have needed to be performed 6–12 weeks after discharge as the peripartum state and active thrombosis can affect these laboratory results (17,18). We were unable to obtain such labs due to loss to follow-up.
In summary, breast infarctive necrosis should be considered in patients with GG and severe breast pain, especially in the setting of underlying hypercoagulable states, such as COVID-19 infection. We describe successful management of infarctive necrosis with pain control and lactation suppression and would recommend further surgical management in an elective setting.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-140/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-140/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-140/coif). KY serves as an unpaid editorial board member of Annals of Breast Surgery from October 2019 to September 2023. AMC reports that she attended a cadaveric education course sponsored by Hologic. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taib NA, Rahmat K. Benign Disorders of the Breast in Pregnancy and Lactation. Adv Exp Med Biol 2020;1252:43-51. [Crossref] [PubMed]
- Vinicki JP, Gonzalez CN, Dubinsky D, et al. Gestational gigantomastia in autoimmune diseases. J Clin Rheumatol 2015;21:110-2. [Crossref] [PubMed]
- Mangla M, Singla D. Gestational Gigantomastia: A Systematic Review of Case Reports. J Midlife Health 2017;8:40-4. [Crossref] [PubMed]
- Rakislova N, Lovane L, Fernandes F, et al. Gestational gigantomastia with fatal outcome. Autops Case Rep 2020;10:e2020213. [Crossref] [PubMed]
- Cabrera C, Radolec M, Prescott A, et al. Interdisciplinary Approach for the Medical Management of Gestational Gigantomastia. AJP Rep 2020;10:e304-8. [Crossref] [PubMed]
- Fletcher MB, Corsini LM, Meyer MD, et al. Gestational gigantomastia: A case report and brief review of the literature. JAAD Case Rep 2020;6:1159-61. [Crossref] [PubMed]
- Strausburg MB, Gehlhausen J, Ludwig K, et al. Diffuse Dermal Angiomatosis of the Breast With an Apparent Etiology of Underlying Calcified Thrombosed Artery With Adjacent Fat Necrosis. Am J Dermatopathol 2016;38:838-41. [Crossref] [PubMed]
- Taboada JL, Stephens TW, Krishnamurthy S, et al. The many faces of fat necrosis in the breast. AJR Am J Roentgenol 2009;192:815-25. [Crossref] [PubMed]
- Davis CE Jr, Wiley WB, Faulconer RJ. Necrosis of the female breast complicating oral anticoagulant treatment. Ann Surg 1972;175:647-56. [Crossref] [PubMed]
- El Khoury M, Mesurolle B, Kao E, et al. Spontaneous thrombosis of pseudoaneurysm of the breast related to core biopsy. AJR Am J Roentgenol 2007;189:W309-11. [Crossref] [PubMed]
- Kichloo A, Dettloff K, Aljadah M, et al. COVID-19 and Hypercoagulability: A Review. Clin Appl Thromb Hemost 2020;26:1076029620962853. [Crossref] [PubMed]
- Hanff TC, Mohareb AM, Giri J, et al. Thrombosis in COVID-19. Am J Hematol 2020;95:1578-89. [Crossref] [PubMed]
- Kraut RY, Brown E, Korownyk C, et al. The impact of breast reduction surgery on breastfeeding: Systematic review of observational studies. PLoS One 2017;12:e0186591. [Crossref] [PubMed]
- Thibaudeau S, Sinno H, Williams B. The effects of breast reduction on successful breastfeeding: a systematic review. J Plast Reconstr Aesthet Surg 2010;63:1688-93. [Crossref] [PubMed]
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov
- Aubey J, Zork N, Sheen JJ. Inpatient obstetric management of COVID-19. Semin Perinatol 2020;44:151280. [Crossref] [PubMed]
- Thomas RH. Hypercoagulability syndromes. Arch Intern Med 2001;161:2433-9. [Crossref] [PubMed]
- Pettker CM, Lockwood CJ. Thromboembolic Disorders in Pregnancy. In: Gabbe SG, Neibyl JR, Simpson JL, et al. editors. Obstetrics: Normal and Problem Pregnancies, Seventh Edition. Philadelphia, PA, USA: Elsevier, 2017:965-80.
Cite this article as: Ecanow NS, Chichura AM, Kopkash K, Pesce C, Sullivan ME, Yao K. Gestational gigantomastia complicated by breast infarctive necrosis in the setting of COVID-19 infection: a case report. Ann Breast Surg 2023;7:30.

