Pre-pectoral implant-based breast reconstruction after mastectomy: a narrative review
Introduction
Breast cancer (BC) is the most frequently diagnosed tumor in women and the leading cause of death from cancer in females (1). More than two million women worldwide are diagnosed with BC every year and up to 40% of these require mastectomy. Furthermore, discovering hereditary factors involved in BC genesis, the benefit of mastectomy in high-risk patients has increased the demand for risk-reducing mastectomy and immediate reconstruction. In fact, about 33.3% of patients between 22 and 44 years old, with unilateral cancer, undergo contralateral risk reduction mastectomy and about 40% of patients with genetic mutations in the BRCA1 and BRCA2 genes decide to undergo bilateral mastectomy (2-5).
The breast is the aesthetic element at the basis of the female image, fundamental to a woman’s psychological and sexual identity and a universal symbol of seduction. The loss of one or both breasts can represent a serious trauma for a woman’s identity with strong impact on the woman’s psychology and relationships (6).
Breast reconstruction after mastectomy is therefore of crucial importance in the view of the physical impairment caused by a demolitive surgery (7). Historically, in the 1970s when the implants were placed directly under the mastectomy flap, there were reported high rates of implant loss (28%), flap necrosis (13.5%) and capsular contracture (56%), which led to the quick abandonment of this technique (8,9). In 1982, Radovan introduced the so-called “two-stage” technique (10) with a tissue expander placed in an “artificially”-made pocket under pectoralis major muscle and serratus anterior fascia in order to have complete muscle coverage at the time of mastectomy. Following an expansion phase, once desired volume had been reached and approximately 4–6 weeks after completion of adjuvant therapy, the second reconstructive time could be carried out, replacing the expander with a definitive implant. The “two-stage” breast reconstruction technique, which has been increasingly perfected over time, has dramatically reduced implant loss, flap necrosis and capsular contracture rates (11-13).
However, even the “two-stage” technique has its disadvantages, including chronic chest pain, physical impairment due to the limited use of the pectoralis major muscle, Breast Animation Deformity (BAD), and discomfort for patients as the subpectoral expander often gives the image of an “unnatural” breast (14,15). In addition, this technique always requires at least two surgeries and two hospitalizations, which is more expensive for the healthcare system.
The history of breast reconstructive surgery has evolved towards becoming increasingly less invasive and the use of direct-to-implant procedures which allow the patient to complete the reconstructive procedure as soon as possible with a rapid return to normal activities. For this purpose, devices called mesh or matrices, have been introduced in order to allow the one-stage immediate breast reconstruction. The term matrix usually refers to products of biological origin, while mesh refers to products in synthetic materials. There is a wide variety of meshes and matrices, which differ in physical properties and composition. The use of acellular dermal matrix (ADM) in breast reconstruction with implants began with Breuing (16) in 2005 and Salzberg (17) in 2006. The authors described the use of the biological matrix in breast implant reconstruction in order to close the inferolateral portion of the subpectoral pocket, thus creating the necessary space for the immediate implantation of the definitive implant, without the need for a tissue expander. Since then, the popularity of meshes and matrices has continued to grow, increasing immediate breast reconstruction with implants from 30% to 50% between 2007 and 2014 (18). Mylvaganam and colleagues (19) showed that, in the UK, 75% of immediate breast reconstructions (IBR) are performed using biological matrices and 24% using synthetic mesh.
Then, surgeons re-introduced the concept of implanting the prosthesis in the pre-pectoral position to reconstruct the breast. With this reconstructive technique, the pectoralis major muscle is left intact on the chest wall and the implant is only covered by the mesh or the matrix and the mastectomy skin flap (20,21). The implant is enveloped in mesh or matrix before being placed under the skin flap or, alternatively, the device is sutured to the fascia of the pectoralis major muscle and then the prosthesis is inserted underneath thus providing only an anterior cover for the implant (22,23).
The technique of breast reconstruction with pre-pectoral implants has been rapidly adopted as an innovative approach in breast reconstruction (24). Avoiding dissection of the pectoralis major muscle means reduced rates of BAD, chronic pain and no loss of muscle function, resulting in improved patient comfort and postoperative functional recovery (25,26). However, results from randomized trials are still not available and literature data mainly derives from large case series.
The aim of this paper is to provide a narrative review of current literature on pre-pectoral breast reconstruction (PBR) after BC over the last five years with a focus on the safety of this procedure in terms of complication rate and the advantages of PBR when compared to submuscular reconstruction Additionally, we reported the results of PBR in the special settings of postmastectomy radiotherapy, revision surgery, skin-sparing mastectomy and hybrid reconstruction. We present the following article in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-147/rc).
Methods
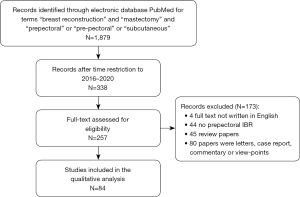
A narrative review of the literature was carried out according to the criteria of Green et al. (27) (Table 1). The electronic database PubMed was searched for studies on pre-pectoral implant-based breast reconstruction using the terms “breast reconstruction” and “mastectomy” and “prepectoral” or “pre-pectoral” or “subcutaneous”. The search was carried out in March 2021 including papers from January 2016 to December 2020. Exclusion criteria were as follows: (I) letters, case reports, reviews, commentary, conference paper and author’s views; (II) studies not written in English; (III) non-human studies.
Table 1
| Items | Specification |
|---|---|
| Date of search | March 1, 2021 |
| Databases and other sources searched | PubMed |
| Search terms used | “breast reconstruction” and “mastectomy” and “prepectoral” or ”pre-pectoral” or “subcutaneous” |
| Timeframe | from January 2016 to December 2020 |
| Exclusion criteria | (I) Letters, case reports, reviews, commentary, conference paper and author’s views |
| (II) Studies not written in English | |
| (III) Non-human studies | |
| Selection process | Independently |
Results
Figure 1 shows the search strategy and literature retrieval workflow. Eighty-four papers were included in this review. Characteristics of the included papers are reported in Table 2.
Table 2
| Study authors | Patients undergoing PBR, No. [%] | Breasts No. [%] | Follow-up [months] | Overall complication rate | Capsular contracture rate (Becker III–IV) | Implant cover | Implant surface | Post-mastectomy RT [%] | DTI or expander [%] |
|---|---|---|---|---|---|---|---|---|---|
| PBR complication profile | |||||||||
| Lo Torto F 2020 (22) | 18 | 22 | 12 [11–15] | 16.7 | 0 | TCPM pocket | NR | NR | TE: 81.8; DTI: 18.2 |
| Kobraei EM 2016 (25) | 13 | 23 | 10 [6–18] | 46 | NR | ADM/Vic mesh | NR | 13 | DTI |
| Jafferbhoy S 2017 (26) | 64 | 78 | 9.98 | 48.4 | NR | ADM | NR | NR | DTI |
| Urquia LN 2020 (28) | 118 | 183 | 9.26 [1–30] | 17.5 | 5.4 | ADM | NR | 29.6 | DTI: 73.1; TE: 26.9 |
| Chandarana M 2020 (29) | 324 | 406 | 9.7 [3–35] | 28.6 | 0.2 | ADM | NR | 15.3 | DTI |
| Downs RK 2016 (30) | 45 | 79 | 23.1±10.4 | 35.0 | 10.1 | ADM | NR | NR | DTI |
| Vidya R 2017 (31) | 51 | 60 | 16.4 [8–25] | 11.6 | NR | ADM | NR | 2.0 | DTI |
| Highton L 2017 (32) | 106 | 166 | 15.3±9.3 | 11.4 | 0 | ADM | NR | 11.8 | DTI: 92.8; TE: 7.2 |
| Woo A 2017 (33) | 79 | 135 | 10 [2–36] | 10.6 | NR | ADM | NR | 0 | DTI: 10; TE: 85 |
| Vidya R 2017 (34) | 79 | 100 | 17.9±3.6 | 13 | NR | ADM | NR | 3.8 | DTI |
| Jones G 2017 (35) | 50 | 73 | 12 [3.2–25.7] | 3 | 1.3 | ADM | NR | 10 | DTI |
| Paydar KZ 2018 (36) | 10 | 18 | 14.1 | 22.2 | 0 | ADM | NR | 10 | DTI: 88.8; TE: 11.2 |
| Jones G 2019 (37) | 234 | 357 | 15.1 | 0.9–44 | 0.9 | ADM | NR | NR | DTI: 68 |
| Chopra K 2019 (38) | 115 | 185 | Least 6 | 32–46 | NR | ADM | – | NR | TE |
| Gabriel A 2019 (39) | 197 | 366 | 21.7±12 | 12 | 1.1 | ADM | NR | NR | DTI: 84.7; TE: 15.3 |
| Momeni A 2019 (40) | 58 | 99 | Nr | 23–42 | NR | ADM | – | 20.6 | TE |
| Kraenzlin FS 2020 (41) | 169 | 267 | At least 12 | 43–60 | NR | ADM | – | 16.0 | TE |
| Safran T 2020 (42) | 201 | 313 | Nr | 17.9 | NR | ADM [77.6] | NR | 18.5 | DTI |
| Casella D 2019 (43) | 187 | 237 | 36.5 [12–72] | 6.7 | 3.8 | TCPM | – | 11 | TE |
| Lee JS 2019 (44) | 23 | NR | 12 | 21.7 | 4.3 | ADM | Textured | 8.7 | DTI |
| Salibian AH 2017 (45) | 155 | 250 | 55.5 [23.6–138] | 0.4–4 | 4 | None | – | 22.0 | TE |
| Sigalove S 2017 (46) | 207 | 353 | 26 | <5 | 0 | ADM | Textured | 8.2 | DTI: 11.1; TE: 85.5 |
| Onesti MG 2017 (47) | 52 | 64 | 24 | 9.6 | 0 | ADM | Smooth | 3.8 | DTI |
| Becker H 2019 (48) | 25 | 37 | Nr | 40 | 4 | Nothing | Smooth | 12 | Spectrum |
| Reitsamer R 2019 (49) | 134 | 200 | 36 [3–68] | 14.5 | 0 | ADM [56.5] versus TIGR mesh [43.5] | Textured | 16 | DTI |
| Nahabedian MY 2020 (50) | 90 | 139 | 21.6 [12–51.6] | NR | 10.1 | ADM | Smooth | 31.1 | TE: 78.9; DTI: 21.1 |
| Woo J 2020 (51) | 21 | 23 | 11.4 | 17 | NR | ADM | Textured | 4.3 | DTI |
| Franck P 2020 (52) | 30 | 66 | 8.3 | 13–20 | NR | Nothing | – | NR | TE |
| Gunnarsson GL 2018 (53) | 27 | 47 | 12 [8.1–17.7] | 14.8 | NR | ADM/Vic mesh | Smooth: 13; Textured: 87 | NR | DTI |
| Manrique OJ 2020 (54) | 40 | 75 | 15 [13.5–17] | 12.5 | 0 | ADM [47.5] | – | 10 | TE |
| Casella D 2019 (55) | 179 | 250 | 38.5 [24–60] | 2.4 | 2 | TCPM | Textured | 10 | DTI |
| Fredman R 2019 (56) | 94 | 153 | 8.5 ±3.9 | 27 | 0 | ADM | Textured: 11; Smooth: 89 | 6 | DTI |
| de Vita R 2019 (57) | 21 | 34 | 4 [2–6] | 4.7 | 0 | Nothing | Polyurethane | NR | DTI |
| Neamonitou F 2020 (58) | 41 | 52 | 14.3 [6–36] | 16 | 2 | ADM | Polyurethane | 29 | DTI |
| Comparison between PBR and submuscular reconstruction | |||||||||
| Potter S 2019 (11) | 42 [2] | 63 [2] | 3 | 26 | NR | ADM: 54; Synthetic mesh: 12 | NR | NR | DTI: 86; TE: 14 |
| Nealon KP 2020 (59) | 114 [44.5] | 183 [43.5] | 19.8±9.9 | 14 | 1.8 | ADM/vic/vic + ADM | NR | 24.6 | DTI |
| Yang JY 2019 (60) | NR | 32 [40.5] | 11.9 | NR | 0 | ADM | NR | 6.3 | DTI: 50; TE: 50 |
| Chandarana MN 2018 (61) | 61 [46.9] | 71 [46] | 9.8 | 5.4 | 1.6 | ADM | NR | 31.1 | DTI |
| Antony AK 2019 (62) | 31 [48.4] | 47 [45.2] | 16.5 | 2 | 0 | ADM | NR | 2 | DTI |
| Thangarajah F 2019 (63) | 34 [54] | NR | 18 | 35.3 | 3 | NR | NR | 8.8 | DTI |
| Sobti N 2020 (64) | 20 [42.5] | 32 [39.5] | 22.9 ± 10 | 10 | 30 | Vic or Vic + ADM | NR | 80 | DTI |
| Mirhaidari SJ 2020 (65) | 62 [48] | 112 [50] | 24 | 19.6 | NR | ADM | NR | 9.6 | DTI |
| Viezel-Mathieu A 2020 (66) | 39 [50.6] | 60 [50.4] | 13.6 [±9.7] | 25 | NR | ADM | Smooth | NR | DTI |
| Manrique OJ 2020 (67) | 33 [44] | 55 [36.6] | 20.3 [12–27] | 7.2 | 0 | ADM | Smooth 33%; Text 67% | 5.5 | DTI |
| Scheflan M 2020 (68) | 49 [38.9] | 71 [40.3] | 18.6 [±13] | 26.8 | 8.5 | ADM | NR | 14.5 | DTI: 77.5; TE: 22.5 |
| Kim JH 2020 (69) | 53 [31.7] | 53 [31.7] | Nr | 37.7 | 3.8 | ADM | NR | 11.3 | DTI |
| Avila A 2020 (70) | 116 [50] | 203 [50.1] | Least 1 | 5.9 | NR | ADM | NR | NR | DTI: 73.9 |
| Baker BG 2018 (71) | 28 [70] | 43 [69.3] | Least 3 | 13.9 | NR | ADM | NR | 0 | DTI: 88.7; TE: 11.3 |
| Cattelani L 2018 (72) | 39 [46.4] | 46 [46.4] | 12 [4–22] | 7.7 | NR | ADM | NR | 13 | DTI |
| Schnarrs RH 2016 (73) | NR | 188 [89] | At least 3 | 19.7 | NR | ADM | – | NR | TE: 98 |
| Bettinger LN 2017 (74) | 110 [51.6] | 165 [56.1] | Minimum 6 | 13.3 | NR | ADM | – | 14 | TE |
| Sbitany H 2017 (75) | 51 [30.7] | 84 [31.1] | 11.1±5.8 | 17.9 | NR | ADM | – | 8.30 | TE |
| Nahabedian MY 2017 (76) | 39 [43.8] | 62 [42.7] | 8.7 [3–21] | 20.5 | 0 | ADM | NR | 30.8 | TE: 90; DTI: 10 |
| Manrique OJ 2019 (77) | 100 [59.1] | 187 [60.1] | 17.9 [12–24] | 10.7 | NR | ADM [99.5] | – | 17.1 | TE |
| Wormer BA 2019 (78) | 32 [31.7] | 60 [32.6] | 6±3.3 | 30 | NR | ADM | – | 21.9 | TE |
| Momeni A 2019 (79) | 40 [50] | 69 [50] | Nr | 32.5 | NR | ADM | – | 20 | TE: 43.6 |
| Gabriel A 2020 (80) | 68 [51.1] | 129 [50.2] | 22.7± 3.5 | 14.7 | 0.8 | ADM | – | NR | TE |
| Braun SE 2020 (81) | 116 [72.5] | 209 [72.5] | 16±8.3 | 24 | NR | ADM [98] | NR | 6.7 | TE: 88; DTI: 12 |
| Walia GS 2018 (82) | 26 [19.2] | NR | Least 2 | 31 | NR | ADM | – | 12 | TE |
| Copeland- Halperin LR 2019 (83) | 94 [61.8] | 160 [62] | 12 | 5.3 | NR | ADM | NR | NR | TE: 60.2; DTI: 39.8 |
| Schaeffer CV 2019 (84) | 24 [33.3] | 45 [33.3] | Least 6 | 21 | NR | ADM | – | NR | TE |
| Radiotherapy after PBR | |||||||||
| Sigalove S 2019 (85) | 33 | 52 | 25.1±6.4 | 5.9 | 0 | ADM | NR | 65.4 | DTI: 36.5; TE: 63.5 |
| Elswick SM 2018 (86) | 54 | 93 | 19 [1–36] | 24.7 | 1.9 | ADM | – | 96.4 | TE |
| Sbitany H 2019 (87) | NR | 175 [42.6] | 9 | NR | NR | ADM | – | 14.9 | TE |
| Sigalove S 2017 (88) | 33 | 52 | 25.1 ± 6.4 | 3.8 | 0 | ADM | NR | 65.4 | DTI: 36.5; TE: 63.5 |
| Sinnott CJ 2018 (89) | 274 [73.2] | 426 [72.3] | 19±16.9 | 12 | 22 | ADM | NR | 56 | DTI |
| Casella D 2019 (90) | 397 | 521 | 38 | 5.8 | 3.6 | TCPM | NR | 15.1 | TE: 47.1; DTI: 52.8 |
| Polotto S 2020 (91) | 160 | 206 | Nr | 25.7 | 1.9 | ADM | Textured | 84.9 | DTI |
| Secondary PBR | |||||||||
| Gabriel A 2018 (92) | 57 | 102 | 16.7 [4–65.8] | 3.9 | 0 | ADM | – | 8.8 | TE |
| Lentz R 2019 (93) | 31 | 55 | 8.3 [1.1–26.9] | 14.5 | 7.3 | ADM | Smooth | NR | DTI |
| Jones GE 2019 (94) | 90 | 142 | 19.2 | 9.1 | 0 | ADM | NR | NR | DTI |
| Sigalove S 2019 (95) | 64 | 124 | 18.9±11 | 3.2 | 0 | ADM + mesh P4HB | Smooth | 4.8 | DTI |
| Holland MC 2020 (96) | 45 | 80 | 15.2 [± 7.14] | 42.5 | 6.3 | ADM | NR | 12.5 | DTI |
| PBR following skin-reducing mastectomy | |||||||||
| Manrique OJ 2020 (97) | 9 | 17 | 23.5 [17–55] | 12 | 0 | ADM | – | NR | TE |
| Caputo GG 2016 (98) | 27 | 33 | 14.7 [6–24] | 11.2 | NR | ADM | Textured | 0 | DTI |
| Becker H 2018 (99) | 20 | 36 | Nr | 45 | NR | Nothing | – | NR | TE |
| Komorowska-Timek E 2019 (100) | 24 [44.4] | 42 [48.2] | 9.6 [0.7–26.4] | 40.5 | 2.7 | Nothing | – | 62.5 | TE |
| Thuman J 2019 (101) | 21 | 37 | 7.76 [3–17] | 30 | NR | ADM [32.4] | Smooth | 33.3 | TE |
| Khalil HH 2019 (102) | 8 | 16 | 12 [3–24] | 0 | NR | ADM | NR | NR | DTI |
| Onesti MG 2020 (103) | 10 | 13 | 31 [24–39] | 10 | 0 | ADM | NR | 0.3 | DTI |
| Maruccia M 2020 (104) | 19 | 23 | 23.2 [±3.4] | 21 | 0 | ADM | Smooth | 13 | DTI |
| Hybrid PBR | |||||||||
| Momeni A 2018 (105) | 23 | 46 | 8.4 [2–17] | 38 | NR | ADM | Smooth | 30.4 | DTI |
| Stillaert FBJL 2020 (106) | 33 | 56 | 24.1 [6–54] | 12 | 0 | None | – | 27 | TE |
| Momeni A 2019 (107) | 31 | 62 | 7.3 [2–12] | 32 | NR | ADM | – | NR | TE |
Spectrum: it is an inflatable breast implant having a detachable filling reservoir, that can be filled under controlled conditions postoperatively. This implant functions either as a tissue expander or as a delayed-filling implant. Once the desired breast size is achieved, the reservoir is removed, leaving the filled implant in position. PBR, pectoral breast reconstruction; RT, radiotherapy; DTI, direct to implant; NR, not reported; ADM, acellular dermal matrix; TCPM, titanium covered polyurethane mesh; TE, tissue expander (or 2 stage reconstruction); vic, vicryl mesh.
The included studies were divided according to the primary outcome of papers into six groups:
- PBR complication profile (34 papers): in this group, there are studies that focused on the feasibility and complication rates of pre-pectoral reconstruction (22,25,26,28-43), including studies with different implants forms and surfaces (44,46,47,49-51,53,55-58); in the end, in this subgroup are present studies without any coverage of implants (neither mesh nor ADM) (45,48,52,54);
- Comparison between PBR and submuscular reconstruction (27 papers): studies comparing pre-pectoral with submuscular reconstruction, both in direct-to-implant (11,59-72) and “two stage” reconstruction (73-84);
- Radiotherapy after PBR (7 papers): studies evaluating the effects of adjuvant radiotherapy on pre-pectoral reconstruction (85-91);
- Secondary PBR (5 papers): studies reporting the conversion to pre-pectoral reconstruction in revision surgeries of previous reconstructed breast (92-96);
- PBR following skin-reducing mastectomy (8 papers): studies reporting patients who underwent skin-reducing mastectomy and PBR (97-104);
- Hybrid PBR (3 papers): studies that focused on breast reconstruction combining autologous tissue transfer with pre-pectoral implant placement (105-107).
PBR complication profile
The most common complications of pre-pectoral reconstruction are (108):
- Seroma: it is usually the most common minor complication and may form after removal of the drain. Some experts say it should always be aspirated, even under ultrasound guidance, while others are more conservative and only aspirate if the seroma is expanding or persistent, as it puts the surgical wound at risk of dehiscence (28,29,44).
- Red breast syndrome (109): a very rare complication whose occurrence can be reduced by washing implants, matrices and meshes before insertion. It should be differentially diagnosed with infection and can be treated conservatively, although it takes several weeks for complete resolution (29,32,47).
- Wound dehiscence/flap necrosis/infection: superficial dehiscence can be managed on an outpatient basis eventually with advanced dressing. Major dehiscence, flap ischaemia and infection require reoperation (30,45,46,110).
- Rippling: an unaesthetic adverse effect of the discussed technique. It is likely to be influenced by the thickness of the mastectomy flap, the type of implant used and the eventual presence of implant coverage (111). It is frequently associated to the visibility of the implant upper pole, which is more evident in underweight patients. In these cases, adipose tissue grafting is the most common corrective procedure for this type of complication (35,37,50).
- Capsular contracture: is one of the most common complications in breast implant surgery, both cosmetic and reconstructive. It has been reported that the risk of experiencing this complication after reconstructive surgery is 12% at one year after surgery, rising to 30% at 5 years after surgery (39,48,112).
In this subgroup, all studies focused on the feasibility of PBR and its complication rate. Although PBR was historically associated to a high complication rate, more recent studies showed that current anatomical and technical knowledge together with modern biocompatible prosthetic materials increasingly allow an acceptable complication rate. In fact, in the largest study population within this subgroup (406 reconstructions performed across 18 centres) (29) the overall complication rate was 28.6%, of which 62 major (15.3%) and 54 minor (13.3%) complications. As regards the management of complications, 51 women (15.7%) needed unplanned readmission and 54 (16.7%) had a surgical exploration within 90 days from the primary operation. Of these, 44 women (13.6%) had a surgical exploration for implant‐related complications and the overall implant loss rate was 6.4%. Of these, 4.9% of implants were removed within 90 days from the primary surgery. Six women had a delayed implant loss, more than 3 months after the reconstruction.
In 2017, Sigalove and colleagues (46) published their preliminary results on 353 pre-pectoral, implant-based, primary reconstructions in 207 patients, of whom 146 were bilateral, following skin- or nipple-sparing mastectomies. In this case series, complications after reconstructive surgery included infection, seroma, and flap necrosis, each occurring at an incidence of less than 5%, additionally there was no capsular contracture. Even when only one surgeon’s experience was described, the complication rate was still acceptable. Similarly, Safran et al. (42) reported a minor complication rate of 9.3% and the major complication rate was 8.6%.
Studies included in this subgroup included different types of implants (anatomical or round) and implant surfaces (smooth or textured), whilst De Vita (57) and Neamonitou (58) used implants with a polyurethane-foam coated surface. The polyurethane-foam coated implants are breast implants coated with micro-polyurethane foam, which minimizes the capsular contracture rate to 0–3% (113-115). The low capsular contracture rate is attributed to the internal growth and microencapsulation of fibroblasts in the polyurethane foam matrix. Unlike implants with both smooth and textured surfaces where a single large capsule is created around, implants covered with micro-polyurethane foam favor the growth of numerous microcapsules around the foam, which is why the contractile forces are neutralised. De Vita et al. (57) reported their preliminary experience with micropolyurethane-foam coated breast implants placed in the pre-pectoral position without any type of covering. The results of 21 patients were encouraging: no major complications, good aesthetic results and excellent patient satisfaction.
Despite the fact that most authors in this subgroup used a coverage device, Manrique et al. (54) reported the Mayo Clinic’s experience on a series of pre-pectoral implant-based breast reconstruction with and without ADM and compared their outcomes. Twenty-one patients reconstructed without the use of meshes had equally good results compared to pre-pectoral reconstruction with mesh, additional to reduced cost and operating time.
Comparison between PBR and submuscular reconstruction
Several cohort studies reported positive outcomes of breast reconstruction with a pre-pectoral implant, with complication rates comparable to that of reconstruction with a subpectoral implant. Braun et al. (81) reported a retrospective series of patients undergoing nipple sparing mastectomy and immediate breast reconstruction in either the pre-pectoral or submuscular plane from January 2015 to June 2019. A total of 288 breasts (160 patients) were included. Overall, the rate of nipple-areola complex necrosis was 15.1%, with no differences between the two cohorts (P=0.79). Similarly, there were no significant differences in overall postoperative complications (P=0.46), including hematoma, seroma, infection, and device exposure.
In 2020, Nealon et al. (59) concluded that pre-pectoral direct-to-implant reconstruction is a safe alternative to subpectoral direct-to-implant reconstruction and that, given the low morbidity and elimination of BAD, it should be considered when the mastectomy skin flap is robust. In fact, their cohort of 114 pre-pectoral versus 142 subpectoral direct-to-implant patients, the results of the penalized regression model demonstrated equivalence in safety metrics including seroma, cancer recurrence, explantation, capsular contracture, mastectomy skin flap necrosis, infection, hematoma, and revision (P>0.05). Similarly, Bettinger et al. (74) demonstrated that pre-pectoral and subpectoral (with or without ADM) breast reconstructions had comparable grade IIIb Clavien score complications. Furthermore, this study showed that BMI >40 kg/m2, stage IV cancer, and contralateral prophylactic mastectomy were associated with adverse expander outcomes and a prior history of radiation therapy adversely impacted implant outcomes.
Overall, PBR can be performed safely and with significantly less pain and earlier return to routine activities compared to submuscular implant placement. In fact, Schaeffer et al. (84) showed that comparing postoperative pain and early functional outcomes between pre-pectoral and partial submuscular breast reconstruction, the first group had significantly lower inpatient pain scores, required significantly fewer intravenous opioids, fewer oral opioids as outpatients, and returned to full active range of shoulder motion in half time. Also, Copeland-Halperin et al. (83) reported a reduced demand for opioids: the pre-pectoral reconstruction group remained for 33% fewer days on opioid analgesic medication (P=0.016) and were 66% less likely to require opioid prescription refills (P=0.027). Therefore, immediate pre-pectoral reconstruction resulted in lower pain intensity and significant upper limb functional advantages in addition to considering a series of ascertained benefits, economically advantageous (72).
To date, further evidence supporting pre-pectoral reconstruction over subpectoral are awaited from ongoing randomized controlled trials (ClinicalTrials.gov Identifier: NCT04293146; ClinicalTrials.gov Identifier: NCT05125991; ClinicalTrials.gov Identifier: NCT04688697; ClinicalTrials.gov Identifier: NCT03959709; ClinicalTrials.gov Identifier: NCT04391296; ClinicalTrials.gov Identifier: NCT04716959). They are going to investigate improvements in arm mobility, quality of life, aesthetic outcomes, complication rate and recurrence risk. Additionally, good quality data will also come from the prospective multicenter cohort study Pre-BRA, setting the basis for a future pragmatic randomized trial (116).
Radiotherapy after PBR
Studies included in this subgroup showed that post-mastectomy radiotherapy appears to be well tolerated in immediate PBR with no great adverse effects. Sbitany et al. (87) reported on 57 breasts receiving postmastectomy radiotherapy and found no difference in complication rates between pre-pectoral and subpectoral implant-based breast reconstruction. In general, the capsular contracture rate after submuscular reconstruction is three times greater and with more severe contractures (Baker grade 3 or 4) than after PBR and adjuvant radiotherapy (89).
In 2019, Casella et al. (90) conducted a retrospective comparative analysis of risk factors and outcomes between patients undergoing direct to-implant and patients undergoing two stages expander-assisted pre-pectoral reconstruction. The binary logistic regression found no significant association between the rate of surgical and aesthetic complications with any other variables considered, in the tissue expander group. Furthermore, in the direct-to-implant group, a significant association was found between surgical complications and BMI and adjuvant radiotherapy. However, the association remained significant only for BMI, when correlated with the aesthetic outcome.
Secondary PBR
PBR is often proposed as a secondary procedure in order to correct BAD and capsular contracture, by changing the implant pocket from underneath to over the muscle. The dramatic improvement in aesthetic outcome with PBR, in particular as regards the reduced animation deformity, improved inframammary fold definition and the postoperative comfort with enhanced shoulder range of motion, triggered an interest in the concept of pre-pectoral conversion as a means of dealing definitively with the problem of animation deformity in the subpectoral patient population. BAD is an almost universal problem that causes patients embarrassment and often discomfort on a daily basis. Previous attempts of ameliorating BAD with fat grafting had mixed results and have never eliminated the deformity at all. Additionally, fat grafting had no impact whatsoever on patient comfort and physical function whilst PBR did. Jones et al. (94) reported their experience with 142 breasts in 90 patients who had undergone elective subpectoral to pre-pectoral implant site conversion. Postoperative resolution of BAD was 100%, the overall complications rates were 4.2% for infection, 2.1% for seroma, and 0.7% for hematoma, dehiscence, partial thickness necrosis, and explantation; therefore, Baker grades II–IV capsular contractures were 0% at 43 months.
A retrospective study including patients who previously had undergone subpectoral (dual plane), implant-based, breast reconstruction and presented for revision reconstruction was published by Sigalove et al. (95). Reasons for revision included animation deformity, pain, asymmetry, implant malposition, size change, capsular contracture, and rippling. A total of 64 patients (124 breasts) met the inclusion criteria and complications occurred in 4 breasts (3.2%), included implant loss (1.6%), seroma (1.6%), hematoma (0.8%), surgical site infection (0.8%), and skin necrosis (0.8%). There was no incidence of capsular contracture, and the presenting complaints were resolved in all cases.
Also, Gabriel et al. (92) solved successfully 102 cases of BAD post-subpectoral implant placement. In this case series, complications occurred in 4 breasts (3.9%) and included seroma (2 breasts), skin necrosis (3 breasts), and wound dehiscence (1 breast). All 4 breasts with complications had their implants removed and replaced. There was no infection or clinically significant capsular contracture, on the contrary, the patient selection was deemed again to be critical for the success of this technique.
PBR following skin-reducing mastectomy
In PBR, a breast prosthesis is placed in the subcutaneous plane, practically replacing the removed breast tissue. Although such an approach represents an attractive strategy in small/moderate breasts, it is not applicable in large and/or severely ptotic breasts, where a skin reduction is required. A Wise mammoplasty pattern with a de-epithelialized dermal sling and submuscular direct-to-implant has been described by Nava et al. (117) to optimize implant-based reconstruction in this patient population. The original technique of immediate breast reconstruction following skin reduction mastectomy was associated with a substantial risk of implant exposure at the inframammary fold. The reason for this complication stemmed from the fact that the inferior portion of the implant was located directly under the inverted “T” incision line, which frequently experiences malperfusion and breaks down (118). The technique was further modified by Bostwick who utilized the deepithelialized inferior breast skin to enhance the coverage of the implant under the troublesome skin juncture (119). In this approach, the superior edge of the deepithelialized skin flap was sutured to the inferior edge of the raised pectoralis major muscle, creating thus a separate well-vascularized implant pocket (119,120). Although the risk of implant exposure decreased with the use of the inferior mastectomy skin flap, the drawbacks associated with subpectoral implant placement persisted. Moreover, although enhanced tissue padding over an implant is frequently desired, additional soft-tissue boost consisting of superiorly located pectoralis muscle does not protect against ischemic complications occurring mostly in the inferior portions of the reconstructed breast.
Therefore, some authors hypothesized that immediate PBR following skin reduction mastectomy could have similar outcomes to the subpectoral counterpart. In fact, Komorowska-Timek et al. (100), compared the complications of pre-pectoral and subpectoral immediate prosthetic breast reconstructions following skin reduction mastectomy in large and ptotic breasts. A total of 54 patients underwent 87 immediate breast reconstructions including 45 subpectoral and 42 pre-pectoral tissue expander placements. The subpectoral patients had more skin flap necrosis (40.0% versus 16.7%, P=0.044) and infections (37.8% versus 11.9%, P=0.01) than their pre-pectoral counterparts, whereas seromas were more common in the pre-pectoral group (4.4% versus 26.2%, P=0.015). The overall complication rate, although higher in the subpectoral group, was not significantly different (62.2% versus 40.5%, P=0.072).
A further advance in the skin-reducing PBR approach was proposed by Caputo et al. (98), who suggested creating a complete pre-pectoral pocket with a dermal flap along with ADM for lower- and upper-pole coverage, respectively, achieving promising results: in only 3 cases (out of a total of 33 breasts), there was skin ischemia, one healed spontaneously, while two patients underwent a minor surgical revision. No implant loss occurred.
Finally, Thuman et al. (101) demonstrated that a pre-pectoral, two-stage breast reconstruction with Wise pattern skin reduction can be a suitable option in patients who have a high BMI.
Hybrid PBR
The hybrid PBR promises to be the next frontier of PBR as it aims at combining the natural effect of autologous reconstruction (i.e., free flaps or fat grafting) with the comfort of a PBR as the transfer of soft tissue allows to reconstruct a natural breast ptosis and the addition of an implant provides the desired projection (105). In particular, this solution could ameliorate the breast profile when the mastectomy flap is too thin and the implant upper pole is visible or the rippling occurs. Stillaert et al. (106) performed 56 hybrid breast reconstructions with good aesthetic outcomes and patient satisfaction showing pleasant breast projection, natural breast motion, and optimal coverage of the pre-pectoral implants. The complication rate was 12.1% and no patients reported capsular contraction, rippling, or major discomfort at a median follow-up of approximately 24 months. Their hybrid approach was based on placing a tissue expander in the first procedure followed by serial sessions of fat grafting to augment the residual autologous (subcutaneous) compartment and the second step foresaw the insertion of a pre-pectoral, ergonomic implant to obtain central core projection and additional volume.
Momeni et al. (105), instead, preferred to combine autologous reconstruction with free flaps and PBR planning a two-step procedure in one or two stages. In detail, they reported results from a retrospective analysis of 23 patients (46 free flaps) who underwent immediate microsurgical breast reconstruction with an autologous free flap and the simultaneous pre-pectoral implant placement. Postoperative complications were acceptable, including hematoma (4.3%), mastectomy skin flap necrosis (21.7%), fat necrosis (13%), and delayed wound healing at the flap donor site (17.4%). Furthermore, in another study (107) they showed that a delayed‐immediate hybrid breast reconstruction improves the ability to match patient expectations related to breast size and that it is associated with a reduction in the rate of mastectomy skin necrosis. This technique consists of a stage 1, in which it is placed bilateral pre-pectoral tissue expander with ADM, and of a stage 2, in which bilateral pre-pectoral expander was replaced with a free abdominal flap with simultaneous silicone gel implant placement, thus obtaining a better patient satisfaction and reduced skin flap necrosis (0%).
Discussion
Breast reconstruction is an essential component in the surgical treatment of women with BC and the main step in ensuring a good quality of life. Good breast reconstruction after mastectomy has a positive impact on the patient’s psychological recovery (121). So far, pre-pectoral breast reconstruction is the easiest way to reconstruct the breast with an implant, as it replaces the missing volume exactly where it was removed. This type of breast reconstruction presents several advantages: the surgical technique is simple and minimally invasive (45), the duration is relatively short (26), blood loss is limited, the muscle function is preserved (32) and the BAD is absent (21). Consequently, the pain is milder, easily controlled (75), in addition to a more rapid recovery time after surgery (30).
Ideal indications for pre-pectoral breast reconstruction are: immediate breast reconstruction, immediate-delayed breast reconstruction following neoadjuvant therapy, delayed breast reconstruction, risk-reducing surgery, breast revision surgery for animation, capsular contracture, breast deformity, muscular problems associated with submuscular implant reconstruction (108).
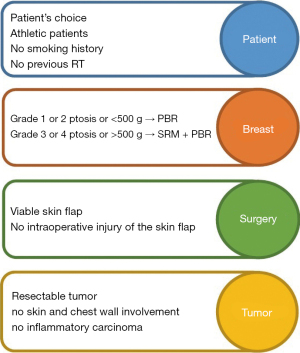
The surgical procedure requires particular attention to specific surgical steps in order to minimise the risk of complications. In fact, patient selection (Figure 2) is crucial for the success of the operation, and the presence of risk factors is associated with an increased risk of adverse events (34). Theoretically, the ideal conditions to choose a PBR have been reported by Vidya et al. (108). The authors reported that this procedure should be offered to patients who are fit and well, with no major or well-controlled comorbidities, body mass BMI index <35 kg/m2, no previous radiotherapy damage, no current smoking, mild ptosis, medium-sized breast (<500 g for one-stage, >500 g for two-stage procedures) and with a resectable tumour not invading skin/chest wall. However, elevated BMI (>40 kg/m2), poorly controlled diabetes mellitus, immunosuppression and previous radiation damage should be considered as relative contraindications (23,46,110). In practice, reviewed studies showed that PBR can be considered for all patients who are candidates for breast reconstruction with implants after mastectomy for cancer or risk reduction, even though risks and benefits must be always well balanced and discussed with the patient (32,59,122). On a technical level, the preservation of the subcutaneous layer of the mastectomy flap and perforator vessels is the key to success. In particular, the vascularity can be assessed intra-operatively by clinical observation (capillary refill time, skin color, texture and temperature, dermal bleeding), or by using special devices such as the SPY Elite System (37,97,104) or other perfusion imaging technology (42). If the flap perfusion is compromised, another type of breast reconstruction should be chosen (30,35). As a result, PBR could be always considered as a reconstructive option in breast surgeon’s armamentarium when a simple natural-looking and fast-recovery procedure is required, but both the surgeon and the patient should be well aware that in patients who do not fulfill the ideal selection criteria the risk of complications is much higher.
Patients can receive antibiotic prophylaxis at the time of induction of anaesthesia (26,35) or postoperatively, antibiotics administered after stratification of infectious risk or according to hospital policy (47,103). Drains should be placed in all patients and removed when the daily output is less than 10–30 mL/day or at the surgeon’s discretion (34). The type of postoperative dressing is determined by the surgeon’s preference (108).
In almost all revised papers, a wide variety of biological matrices and synthetic meshes have been used. The use of meshes and matrices has allowed better control of the implant position, a better definition of the inframammary fold, a reduction in the capsular contracture rate due to a reduced inflammatory response (30,123), and in general, an improvement in aesthetic results (113,114,124). Matrices have also been used in the ‘two-stage’ reconstructions with tissue expander (115,125), allowing a greater initial expander filling and faster expansion. However, a systematic review has shown that breast reconstruction with mesh and matrices significantly increases the rates of seroma, infection and reconstruction failure, compared to procedures without these devices (126). In a further study of 415 reconstructions with implants, the risk of infection was shown to increase 5-fold with the use of ADM (127). Furthermore, the use of no ADM reduces costs and operative time (54,57). As a result, some authors do not use any device to cover the implant or expander in PBR (45,48,52,54,57,99,100). In particular, De Vita et al. (57) used implants with micropolyurethane surface, which represent an integrated ready-to-use device without need further coverage, obtaining the benefits of a coverage (greater biocompatibility, increase in the thickness of the flap), but using only one device (reduced cost and operative time). Other authors, instead, positioned implant or expander without any coverage directly under the skin flap. Their data demonstrated no significant differences in aesthetic scores and in postoperative complications. Therefore, this alternative technique has shown promising results, although data available, follow-up duration and sample size are still limited to derive strong recommendations. However, waiting for stronger evidence, we are not able to define whether the ADM will fall into disuse.
The strength of this study is evident by the supporting fonts of comprehensive data regarding a relatively new technique that has emerged and spread in the last five years, focusing on special issues related to this procedure. The complication profile, the comparison with the standard two-stage breast reconstruction, radiotherapy issues, the option for revision surgery and the possibility of PBR after skin-sparing mastectomy or like a hybrid operation represent fields of great interest for the breast surgeon who applies or is going to start applying this technique. However, the main limitation of this review is that it is not systematic and includes only studies from 2016 to 2020, therefore the available follow-up is often relatively short.
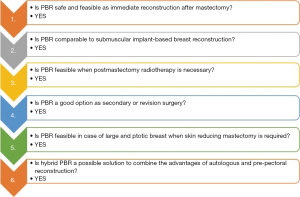
To summarize, reviewed studies showed that (Figure 3):
- PBR, despite being a relatively new technique, has rapidly become widespread and numerous case series have been published. The evidence available demonstrates that PBR is safe and feasible with the use of meshes but they could eventually be abandoned in the future if preliminary data will be confirmed.
- The complication profile is not inferior to subpectoral reconstruction and is absolutely better in terms of pain and animation deformity, ongoing randomized controlled trials will give further evidence about this.
- Regarding the special setting of post-mastectomy radiotherapy after PBR, it is well tolerated in immediate PBR with no great adverse effects, in fact, the capsular contracture rate is three less great than after submuscular reconstruction and radiotherapy.
- PBR could be a useful and good alternative to standard subpectoral reconstruction in case of suboptimal aesthetic results or complications or patient unsatisfaction instead of recurring of opting for autologous reconstruction.
- The application of the skin reducing mastectomy pattern to PBR allows extending the indication to PBR to patients having large and ptotic breasts with results comparable to subpectoral reconstruction also providing a dermal sling to cover the inferior pole in place of meshes.
- Hybrid PBR looks at the future combining the natural effect of autologous reconstruction with the comfort of a PBR. It could allow correcting common complications after PBR like rippling or the visibility of implant upper pole by improving the breast profile. However, data is still too limited to derive meaningful conclusions.
Conclusions
Until a few years ago, subpectoral implant placement was undoubtedly the gold standard for implant-based breast reconstruction. Recently, considerable attention has been paid to performing immediate and delayed reconstruction with the implant in the pre-pectoral position thus overcoming many of the complications associated with subpectoral implant positioning. Although minor complications are quite frequent after PBR, they could be managed conservatively and this remains a promising technique, which gives women natural-looking breasts immediately after mastectomy. The procedure is technically simple minimally invasive, has an acceptable complication rate, and is becoming more and more popular. Although there are ideal candidates, indications can be extended also in cases of relative contraindications while still obtaining a safe complication profile even in special settings such as: when postmastectomy radiotherapy is needed, as a secondary procedure after a previously failed or complicated implant reconstruction, in large or ptotic breast requiring ski-reducing mastectomy or in combination with autologous reconstruction in order to ameliorate the breast profile.
To date, PBR should definitely be included in the breast surgeon’s armamentarium of reconstructive procedures as it offers a relatively simple and quick one-step solution to restore the breast immediately after mastectomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nicola Rocco, Giacomo Montagna and Giuseppe Catanuto) for the series “New Perspectives in Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-147/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-147/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-147/coif). The series “New Perspectives in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. ODG receives consulting fees from MSD, AstraZeneca, Bayer, BD. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Anders CK, Johnson R, Litton J, et al. Breast cancer before age 40 years. Semin Oncol 2009;36:237-49. [Crossref] [PubMed]
- Nash R, Goodman M, Lin CC, et al. State Variation in the Receipt of a Contralateral Prophylactic Mastectomy Among Women Who Received a Diagnosis of Invasive Unilateral Early-Stage Breast Cancer in the United States, 2004-2012. JAMA Surg 2017;152:648-57. [Crossref] [PubMed]
- Henry DA, Lee MC, Almanza D, et al. Trends in use of bilateral prophylactic mastectomy vs high-risk surveillance in unaffected carriers of inherited breast cancer syndromes in the Inherited Cancer Registry (ICARE). Breast Cancer Res Treat 2019;174:39-45. [Crossref] [PubMed]
- Malata CM, McIntosh SA, Purushotham AD. Immediate breast reconstruction after mastectomy for cancer. Br J Surg 2000;87:1455-72. [Crossref] [PubMed]
- Pelusi J. Sexuality and body image. Research on breast cancer survivors documents altered body image and sexuality. Am J Nurs 2006;106:32-8. [Crossref] [PubMed]
- Cataliotti L, Costa A, Daly PA, et al. Florence statement on breast cancer, 1998 forging the way ahead for more research on and better care in breast cancer. Eur J Cancer 1999;35:14-5. [Crossref] [PubMed]
- Snyderman RK, Guthrie RH. Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg 1971;47:565-7. [Crossref] [PubMed]
- Schlenker JD, Bueno RA, Ricketson G, et al. Loss of silicone implants after subcutaneous mastectomy and reconstruction. Plast Reconstr Surg 1978;62:853-61. [Crossref] [PubMed]
- Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg 1982;69:195-208. [Crossref] [PubMed]
- Potter S, Conroy EJ, Cutress RI, et al. Short-term safety outcomes of mastectomy and immediate implant-based breast reconstruction with and without mesh (iBRA): a multicentre, prospective cohort study. Lancet Oncol 2019;20:254-66. [Crossref] [PubMed]
- Scarpa C, Borso GF, Vindigni V, et al. Polyurethane foam-covered breast implants: a justified choice? Eur Rev Med Pharmacol Sci 2015;19:1600-6. [PubMed]
- Duxbury PJ, Harvey JR. Systematic review of the effectiveness of polyurethane-coated compared with textured silicone implants in breast surgery. J Plast Reconstr Aesthet Surg 2016;69:452-60. [Crossref] [PubMed]
- Rohrich RJ, Adams WP Jr, Beran SJ, et al. An analysis of silicone gel-filled breast implants: diagnosis and failure rates. Plast Reconstr Surg 1998;102:2304-8; discussion 2309. [Crossref] [PubMed]
- Serletti JM, Fosnot J, Nelson JA, et al. Breast reconstruction after breast cancer. Plast Reconstr Surg 2011;127:124e-35e. [Crossref] [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [Crossref] [PubMed]
- Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006;57:1-5. [Crossref] [PubMed]
- Mennie JC, Mohanna PN, O'Donoghue JM, et al. National trends in immediate and delayed post-mastectomy reconstruction procedures in England: A seven-year population-based cohort study. Eur J Surg Oncol 2017;43:52-61. [Crossref] [PubMed]
- Mylvaganam S, Conroy E, Williamson PR, et al. Variation in the provision and practice of implant-based breast reconstruction in the UK: Results from the iBRA national practice questionnaire. Breast 2017;35:182-90. [Crossref] [PubMed]
- Casella D, Bernini M, Bencini L, et al. TiLoop® Bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur J Plast Surg 2014;37:599-604. [Crossref] [PubMed]
- Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 2015;68:162-7. [Crossref] [PubMed]
- Lo Torto F, Marcasciano M, Kaciulyte J, et al. Prepectoral breast reconstruction with TiLoop® Bra Pocket: a single center prospective study. Eur Rev Med Pharmacol Sci 2020;24:991-9. [PubMed]
- Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous Direct-to-Implant Breast Reconstruction: Surgical, Functional, and Aesthetic Results after Long-Term Follow-Up. Plast Reconstr Surg Glob Open 2016;3:e574. [Crossref] [PubMed]
- Vidya R. Prepectoral Breast Reconstruction or Muscle-Sparing Technique with the Braxon Porcine Acellular Dermal Matrix. Plast Reconstr Surg Glob Open 2017;5:e1364. [Crossref] [PubMed]
- Kobraei EM, Cauley R, Gadd M, et al. Avoiding Breast Animation Deformity with Pectoralis-Sparing Subcutaneous Direct-to-Implant Breast Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e708. [Crossref] [PubMed]
- Jafferbhoy S, Chandarana M, Houlihan M, et al. Early multicentre experience of pre-pectoral implant based immediate breast reconstruction using Braxon. Gland Surg 2017;6:682-8. [Crossref] [PubMed]
- Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med 2006;5:101-17. [Crossref] [PubMed]
- Urquia LN, Hart AM, Liu DZ, et al. Surgical Outcomes in Prepectoral Breast Reconstruction. Plast Reconstr Surg Glob Open 2020;8:e2744. [Crossref] [PubMed]
- Chandarana M, Harries SNational Braxon Audit Study Group. Multicentre study of prepectoral breast reconstruction using acellular dermal matrix. BJS Open 2020;4:71-7. [Crossref] [PubMed]
- Downs RK, Hedges K. An Alternative Technique for Immediate Direct-to-Implant Breast Reconstruction-A Case Series. Plast Reconstr Surg Glob Open 2016;4:e821. [Crossref] [PubMed]
- Vidya R, Cawthorn SJ. Muscle-Sparing ADM-Assisted Breast Reconstruction Technique Using Complete Breast Implant Coverage: A Dual-Institute UK-Based Experience. Breast Care (Basel) 2017;12:251-4. [Crossref] [PubMed]
- Highton L, Johnson R, Kirwan C, et al. Prepectoral Implant-Based Breast Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1488. [Crossref] [PubMed]
- Woo A, Harless C, Jacobson SR. Revisiting an Old Place: Single-Surgeon Experience on Post-Mastectomy Subcutaneous Implant-Based Breast Reconstruction. Breast J 2017;23:545-53. [Crossref] [PubMed]
- Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: First multicenter European report on 100 cases. Breast J 2017;23:670-6. [Crossref] [PubMed]
- Jones G, Yoo A, King V, et al. Prepectoral Immediate Direct-to-Implant Breast Reconstruction with Anterior AlloDerm Coverage. Plast Reconstr Surg 2017;140:31S-8S. [Crossref] [PubMed]
- Paydar KZ, Wirth GA, Mowlds DS. Prepectoral Breast Reconstruction with Fenestrated Acellular Dermal Matrix: A Novel Design. Plast Reconstr Surg Glob Open 2018;6:e1712. [Crossref] [PubMed]
- Jones G, Antony AK. Single stage, direct to implant pre-pectoral breast reconstruction. Gland Surg 2019;8:53-60. [Crossref] [PubMed]
- Chopra K, Singh D, Hricz N, et al. Two-stage Prosthetic Prepectoral Breast Reconstruction: Comparing Tissue Expansion with Carbon Dioxide and Saline. Plast Reconstr Surg Glob Open 2019;7:e2051. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Sigalove NM, et al. Effect of Body Mass Index on Outcomes after Prepectoral Breast Reconstruction. Plast Reconstr Surg 2019;144:550-8. [Crossref] [PubMed]
- Momeni A, Li AY, Tsai J, et al. The Impact of Device Innovation on Clinical Outcomes in Expander-based Breast Reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2524. [Crossref] [PubMed]
- Kraenzlin FS, Darrach H, Chopra K, et al. Prepectoral 2-stage Breast Reconstruction with Carbon Dioxide Tissue Expansion. Plast Reconstr Surg Glob Open 2020;8:e2850. [Crossref] [PubMed]
- Safran T, Al-Halabi B, Viezel-Mathieu A, et al. Direct-to-Implant, Prepectoral Breast Reconstruction: A Single-Surgeon Experience with 201 Consecutive Patients. Plast Reconstr Surg 2020;145:686e-96e. [Crossref] [PubMed]
- Casella D, Di Taranto G, Marcasciano M, et al. Subcutaneous expanders and synthetic mesh for breast reconstruction: Long-term and patient-reported BREAST-Q outcomes of a single-center prospective study. J Plast Reconstr Aesthet Surg 2019;72:805-12. [Crossref] [PubMed]
- Lee JS, Kim JS, Lee JH, et al. Prepectoral breast reconstruction with complete implant coverage using double-crossed acellular dermal matrixs. Gland Surg 2019;8:748-57. [Crossref] [PubMed]
- Salibian AH, Harness JK, Mowlds DS. Staged Suprapectoral Expander/Implant Reconstruction without Acellular Dermal Matrix following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;139:30-9. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Onesti MG, Maruccia M, Di Taranto G, et al. Clinical, histological, and ultrasound follow-up of breast reconstruction with one-stage muscle-sparing "wrap" technique: A single-center experience. J Plast Reconstr Aesthet Surg 2017;70:1527-36. [Crossref] [PubMed]
- Becker H, Mathew PJ. Immediate Prepectoral Breast Reconstruction in Suboptimal Patients Using an Air-filled Spacer. Plast Reconstr Surg Glob Open 2019;7:e2470. [Crossref] [PubMed]
- Reitsamer R, Peintinger F, Klaassen-Federspiel F, et al. Prepectoral direct-to-implant breast reconstruction with complete ADM or synthetic mesh coverage - 36-Months follow-up in 200 reconstructed breasts. Breast 2019;48:32-7. [Crossref] [PubMed]
- Nahabedian MY. What Are the Long-Term Aesthetic Issues in Prepectoral Breast Reconstruction? Aesthet Surg J 2020;40:S29-37. [Crossref] [PubMed]
- Woo J, Seung IH, Hong SE. Funnel usefulness in direct-to-implant breast reconstruction using periareolar incision with prepectoral implant placement and complete coverage with acellular dermal matrix. J Plast Reconstr Aesthet Surg 2020;73:2016-24. [Crossref] [PubMed]
- Franck P, Chadab T, Poveromo L, et al. Prepectoral Dual-Port Tissue Expander Placement: Can This Eliminate Suction Drain Use? Ann Plast Surg 2020;85:S60-2. [Crossref] [PubMed]
- Gunnarsson GL, Thomsen JB. Prepectoral Hammock and Direct-to-implant Breast Reconstruction in 10 Minutes: A Focus on Technique. Plast Reconstr Surg Glob Open 2018;6:e1931. [Crossref] [PubMed]
- Manrique OJ, Huang TC, Martinez-Jorge J, et al. Prepectoral Two-Stage Implant-Based Breast Reconstruction with and without Acellular Dermal Matrix: Do We See a Difference? Plast Reconstr Surg 2020;145:263e-72e. [Crossref] [PubMed]
- Casella D, Di Taranto G, Marcasciano M, et al. Evaluation of Prepectoral Implant Placement and Complete Coverage with TiLoop Bra Mesh for Breast Reconstruction: A Prospective Study on Long-Term and Patient-Reported BREAST-Q Outcomes. Plast Reconstr Surg 2019;143:1e-9e. [Crossref] [PubMed]
- Fredman R, Wu C, Rapolti M, et al. Prepectoral Direct-to-Implant Breast Reconstruction: Early Outcomes and Analysis of Postoperative Pain. Aesthet Surg J Open Forum 2019;1:ojz006. [Crossref] [PubMed]
- de Vita R, Buccheri EM, Villanucci A, et al. Breast Reconstruction Actualized in Nipple-sparing Mastectomy and Direct-to-implant, Prepectoral Polyurethane Positioning: Early Experience and Preliminary Results. Clin Breast Cancer 2019;19:e358-63. [Crossref] [PubMed]
- Neamonitou F, Mylvaganam S, Salem F, et al. Outcome of complete acellular dermal matrix wrap with polyurethane implant in immediate prepectoral breast reconstruction. Arch Plast Surg 2020;47:567-73. [Crossref] [PubMed]
- Nealon KP, Weitzman RE, Sobti N, et al. Prepectoral Direct-to-Implant Breast Reconstruction: Safety Outcome Endpoints and Delineation of Risk Factors. Plast Reconstr Surg 2020;145:898e-908e. [Crossref] [PubMed]
- Yang JY, Kim CW, Lee JW, et al. Considerations for patient selection: Prepectoral versus subpectoral implant-based breast reconstruction. Arch Plast Surg 2019;46:550-7. [Crossref] [PubMed]
- Chandarana MN, Jafferbhoy S, Marla S, et al. Acellular dermal matrix in implant-based immediate breast reconstructions: a comparison of prepectoral and subpectoral approach. Gland Surg 2018;7:S64-9. [Crossref] [PubMed]
- Antony AK, Poirier J, Madrigrano A, et al. Evolution of the Surgical Technique for "Breast in a Day" Direct-to-Implant Breast Reconstruction: Transitioning from Dual-Plane to Prepectoral Implant Placement. Plast Reconstr Surg 2019;143:1547-56. [Crossref] [PubMed]
- Thangarajah F, Treeter T, Krug B, et al. Comparison of Subpectoral versus Prepectoral Immediate Implant Reconstruction after Skin- and Nipple-Sparing Mastectomy in Breast Cancer Patients: A Retrospective Hospital-Based Cohort Study. Breast Care (Basel) 2019;14:382-7. [Crossref] [PubMed]
- Sobti N, Weitzman RE, Nealon KP, et al. Evaluation of capsular contracture following immediate prepectoral versus subpectoral direct-to-implant breast reconstruction. Sci Rep 2020;10:1137. [Crossref] [PubMed]
- Mirhaidari SJ, Azouz V, Wagner DS. Prepectoral Versus Subpectoral Direct to Implant Immediate Breast Reconstruction. Ann Plast Surg 2020;84:263-70. [Crossref] [PubMed]
- Viezel-Mathieu A, Alnaif N, Aljerian A, et al. Acellular Dermal Matrix-sparing Direct-to-implant Prepectoral Breast Reconstruction: A Comparative Study Including Cost Analysis. Ann Plast Surg 2020;84:139-43. [Crossref] [PubMed]
- Manrique OJ, Kapoor T, Banuelos J, et al. Single-Stage Direct-to-Implant Breast Reconstruction: A Comparison Between Subpectoral Versus Prepectoral Implant Placement. Ann Plast Surg 2020;84:361-5. [Crossref] [PubMed]
- Scheflan M, Allweis TM, Ben Yehuda D, et al. Meshed Acellular Dermal Matrix in Immediate Prepectoral Implant-based Breast Reconstruction. Plast Reconstr Surg Glob Open 2020;8:e3265. [Crossref] [PubMed]
- Kim JH, Hong SE. A Comparative Analysis between Subpectoral versus Prepectoral Single Stage Direct-to-Implant Breast Reconstruction. Medicina (Kaunas) 2020;56:537. [Crossref] [PubMed]
- Avila A, Bartholomew AJ, Sosin M, et al. Acute Postoperative Complications in Prepectoral versus Subpectoral Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2020;146:715e-20e. [Crossref] [PubMed]
- Baker BG, Irri R, MacCallum V, et al. A Prospective Comparison of Short-Term Outcomes of Subpectoral and Prepectoral Strattice-Based Immediate Breast Reconstruction. Plast Reconstr Surg 2018;141:1077-84. [Crossref] [PubMed]
- Cattelani L, Polotto S, Arcuri MF, et al. One-Step Prepectoral Breast Reconstruction With Dermal Matrix-Covered Implant Compared to Submuscular Implantation: Functional and Cost Evaluation. Clin Breast Cancer 2018;18:e703-11. [Crossref] [PubMed]
- Schnarrs RH, Carman CM, Tobin C, et al. Complication Rates With Human Acellular Dermal Matrices: Retrospective Review of 211 Consecutive Breast Reconstructions. Plast Reconstr Surg Glob Open 2016;4:e1118. [Crossref] [PubMed]
- Bettinger LN, Waters LM, Reese SW, et al. Comparative Study of Prepectoral and Subpectoral Expander-Based Breast Reconstruction and Clavien IIIb Score Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1433. [Crossref] [PubMed]
- Sbitany H, Piper M, Lentz R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;140:432-43. [Crossref] [PubMed]
- Nahabedian MY, Cocilovo C. Two-Stage Prosthetic Breast Reconstruction: A Comparison Between Prepectoral and Partial Subpectoral Techniques. Plast Reconstr Surg 2017;140:22S-30S. [Crossref] [PubMed]
- Manrique OJ, Banuelos J, Abu-Ghname A, et al. Surgical Outcomes of Prepectoral Versus Subpectoral Implant-based Breast Reconstruction in Young Women. Plast Reconstr Surg Glob Open 2019;7:e2119. [Crossref] [PubMed]
- Wormer BA, Valmadrid AC, Ganesh Kumar N, et al. Reducing Expansion Visits in Immediate Implant-Based Breast Reconstruction: A Comparative Study of Prepectoral and Subpectoral Expander Placement. Plast Reconstr Surg 2019;144:276-86. [Crossref] [PubMed]
- Momeni A, Remington AC, Wan DC, et al. A Matched-Pair Analysis of Prepectoral with Subpectoral Breast Reconstruction: Is There a Difference in Postoperative Complication Rate? Plast Reconstr Surg 2019;144:801-7. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Storm-Dickerson TL, et al. Dual-Plane versus Prepectoral Breast Reconstruction in High-Body Mass Index Patients. Plast Reconstr Surg 2020;145:1357-65. [Crossref] [PubMed]
- Braun SE, Dreicer M, Butterworth JA, et al. Do Nipple Necrosis Rates Differ in Prepectoral Versus Submuscular Implant-Based Reconstruction After Nipple-Sparing Mastectomy? Ann Surg Oncol 2020;27:4760-6. [Crossref] [PubMed]
- Walia GS, Aston J, Bello R, et al. Prepectoral Versus Subpectoral Tissue Expander Placement: A Clinical and Quality of Life Outcomes Study. Plast Reconstr Surg Glob Open 2018;6:e1731. [Crossref] [PubMed]
- Copeland-Halperin LR, Yemc L, Emery E, et al. Evaluating Postoperative Narcotic Use in Prepectoral Versus Dual-plane Breast Reconstruction Following Mastectomy. Plast Reconstr Surg Glob Open 2019;7:e2082. [Crossref] [PubMed]
- Schaeffer CV, Dassoulas KR, Thuman J, et al. Early Functional Outcomes After Prepectoral Breast Reconstruction: A Case-Matched Cohort Study. Ann Plast Surg 2019;82:S399-403. [Crossref] [PubMed]
- Sigalove S. Prepectoral breast reconstruction and radiotherapy-a closer look. Gland Surg 2019;8:67-74. [Crossref] [PubMed]
- Elswick SM, Harless CA, Bishop SN, et al. Prepectoral Implant-Based Breast Reconstruction with Postmastectomy Radiation Therapy. Plast Reconstr Surg 2018;142:1-12. [Crossref] [PubMed]
- Sbitany H, Gomez-Sanchez C, Piper M, et al. Prepectoral Breast Reconstruction in the Setting of Postmastectomy Radiation Therapy: An Assessment of Clinical Outcomes and Benefits. Plast Reconstr Surg 2019;143:10-20. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction and Postmastectomy Radiotherapy: Short-Term Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1631. [Crossref] [PubMed]
- Sinnott CJ, Persing SM, Pronovost M, et al. Impact of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann Surg Oncol 2018;25:2899-908. [Crossref] [PubMed]
- Casella D, Di Taranto G, Onesti MG, et al. A retrospective comparative analysis of risk factors and outcomes in direct-to-implant and two-stages prepectoral breast reconstruction: BMI and radiotherapy as new selection criteria of patients. Eur J Surg Oncol 2019;45:1357-63. [Crossref] [PubMed]
- Polotto S, Bergamini ML, Pedrazzi G, et al. One-step prepectoral breast reconstruction with porcine dermal matrix-covered implant: a protective technique improving the outcome in post-mastectomy radiation therapy setting. Gland Surg 2020;9:219-28. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral Revision Breast Reconstruction for Treatment of Implant-Associated Animation Deformity: A Review of 102 Reconstructions. Aesthet Surg J 2018;38:519-26. [Crossref] [PubMed]
- Lentz R, Alcon A, Sbitany H. Correction of animation deformity with subpectoral to prepectoral implant exchange. Gland Surg 2019;8:75-81. [Crossref] [PubMed]
- Jones GE, King VA, Yoo A. Prepectoral Site Conversion for Animation Deformity. Plast Reconstr Surg Glob Open 2019;7:e2301. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Gabriel A. Outcomes Utilizing Inspira Implants in Revisionary Reconstructive Surgery. Plast Reconstr Surg 2019;144:66S-72S. [Crossref] [PubMed]
- Holland MC, Lentz R, Sbitany H. Surgical Correction of Breast Animation Deformity with Implant Pocket Conversion to a Prepectoral Plane. Plast Reconstr Surg 2020;145:632-42. [Crossref] [PubMed]
- Manrique OJ, Arif C, Banuelos J, et al. Prepectoral Breast Reconstruction in Nipple-Sparing Mastectomy With Immediate Mastopexy. Ann Plast Surg 2020;85:18-23. [Crossref] [PubMed]
- Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-Reduction Breast Reconstructions with Prepectoral Implant. Plast Reconstr Surg 2016;137:1702-5. [Crossref] [PubMed]
- Becker H, Zhadan O. Tissue Contraction-A New Paradigm in Breast Reconstruction. Plast Reconstr Surg Glob Open 2018;6:e1865. [Crossref] [PubMed]
- Komorowska-Timek E, Merrifield B, Turfe Z, et al. Subcutaneous Prosthetic Breast Reconstructions following Skin Reduction Mastectomy. Plast Reconstr Surg Glob Open 2019;7:e2078. [Crossref] [PubMed]
- Thuman J, Freitas AM, Schaeffer C, et al. Prepectoral Wise-Pattern Staged Implant-Based Breast Reconstruction for Obese or Ptotic Patients. Ann Plast Surg 2019;82:S404-9. [Crossref] [PubMed]
- Khalil HH, Malahias MN, Youssif S, et al. Nipple-Sparing Mastectomy and Prepectoral Implant/Acellular Dermal Matrix Wrap Reconstruction in Large Ptotic Breasts. Plast Reconstr Surg Glob Open 2019;7:e2289. [Crossref] [PubMed]
- Onesti MG, Di Taranto G, Ribuffo D, et al. ADM-assisted prepectoral breast reconstruction and skin reduction mastectomy: Expanding the indications for subcutaneous reconstruction. J Plast Reconstr Aesthet Surg 2020;73:673-80. [Crossref] [PubMed]
- Maruccia M, Elia R, Gurrado A, et al. Skin-Reducing Mastectomy and Pre-pectoral Breast Reconstruction in Large Ptotic Breasts. Aesthetic Plast Surg 2020;44:664-72. [Crossref] [PubMed]
- Momeni A, Kanchwala S. Hybrid Prepectoral Breast Reconstruction: A Surgical Approach that Combines the Benefits of Autologous and Implant-Based Reconstruction. Plast Reconstr Surg 2018;142:1109-15. [Crossref] [PubMed]
- Stillaert FBJL, Lannau B, Van Landuyt K, et al. The Prepectoral, Hybrid Breast Reconstruction: The Synergy of Lipofilling and Breast Implants. Plast Reconstr Surg Glob Open 2020;8:e2966. [Crossref] [PubMed]
- Momeni A, Kanchwala S. Delayed-immediate hybrid breast reconstruction-Increasing patient input and precision in breast reconstruction. Breast J 2019;25:898-902. [Crossref] [PubMed]
- Vidya R, Berna G, Sbitany H, et al. Prepectoral implant-based breast reconstruction: a joint consensus guide from UK, European and USA breast and plastic reconstructive surgeons. Ecancermedicalscience 2019;13:927. [Crossref] [PubMed]
- Nahabedian MY. Prosthetic Breast Reconstruction and Red Breast Syndrome: Demystification and a Review of the Literature. Plast Reconstr Surg Glob Open 2019;7:e2108. [Crossref] [PubMed]
- Casella D, Calabrese C, Bianchi S, et al. Subcutaneous Tissue Expander Placement with Synthetic Titanium-Coated Mesh in Breast Reconstruction: Long-term Results. Plast Reconstr Surg Glob Open 2016;3:e577. [Crossref] [PubMed]
- Tasoulis MK, Iqbal FM, Cawthorn S, et al. Subcutaneous implant breast reconstruction: Time to reconsider? Eur J Surg Oncol 2017;43:1636-46. [Crossref] [PubMed]
- Handel N, Cordray T, Gutierrez J, et al. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg 2006;117:757-67; discussion 768-72. [Crossref] [PubMed]
- Vu MM, Kim JY. Current opinions on indications and algorithms for acellular dermal matrix use in primary prosthetic breast reconstruction. Gland Surg 2015;4:195-203. [PubMed]
- Zhong T, Temple-Oberle C, Hofer SO, et al. The Multi Centre Canadian Acellular Dermal Matrix Trial (MCCAT): study protocol for a randomized controlled trial in implant-based breast reconstruction. Trials 2013;14:356. [Crossref] [PubMed]
- Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:1049-58. [Crossref] [PubMed]
- Harvey KL, Mills N, White P, et al. The Pre-BRA (pre-pectoral Breast Reconstruction EvAluation) feasibility study: protocol for a mixed-methods IDEAL 2a/2b prospective cohort study to determine the safety and effectiveness of prepectoral implant-based breast reconstruction. BMJ Open 2020;10:e033641. [Crossref] [PubMed]
- Nava MB, Cortinovis U, Ottolenghi J, et al. Skin-reducing mastectomy. Plast Reconstr Surg 2006;118:603-10; discussion 611-3. [Crossref] [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L, et al. Inverted-T skin-reducing mastectomy with immediate implant reconstruction using the submuscular-subfascial pocket. Plast Reconstr Surg 2012;130:31-41. [Crossref] [PubMed]
- Carlson GW, Bostwick J 3rd, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg 1997;225:570-5; discussion 575-8. [Crossref] [PubMed]
- Hammond DC, Capraro PA, Ozolins EB, et al. Use of a skin-sparing reduction pattern to create a combination skin-muscle flap pocket in immediate breast reconstruction. Plast Reconstr Surg 2002;110:206-11. [Crossref] [PubMed]
- Eltahir Y, Werners LLCH, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg 2013;132:201e-9e. [Crossref] [PubMed]
- Becker H, Lind JG 2nd, Hopkins EG. Immediate Implant-based Prepectoral Breast Reconstruction Using a Vertical Incision. Plast Reconstr Surg Glob Open 2015;3:e412. [Crossref] [PubMed]
- Mowlds DS, Salibian AA, Scholz T, et al. Capsular Contracture in Implant-Based Breast Reconstruction: Examining the Role of Acellular Dermal Matrix Fenestrations. Plast Reconstr Surg 2015;136:629-35. [Crossref] [PubMed]
- Nahabedian MY, Spear SL. Acellular dermal matrix for secondary procedures following prosthetic breast reconstruction. Aesthet Surg J 2011;31:38S-50S. [Crossref] [PubMed]
- Kim JYS, Connor CM. Focus on technique: two-stage implant-based breast reconstruction. Plast Reconstr Surg 2012;130:104S-15S. [Crossref] [PubMed]
- Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg 2012;68:346-56. [Crossref] [PubMed]
- Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429-36. [Crossref] [PubMed]
Cite this article as: Di Micco R, Santurro L, Lapiana G, Socci D, Zuber V, Cisternino G, Baleri S, Rottino S, Ceccarino R, Gentilini OD. Pre-pectoral implant-based breast reconstruction after mastectomy: a narrative review. Ann Breast Surg 2023;7:27.