The role of the geriatrician in the care of older patients with breast cancer: a review
Introduction
Due to the aging of the population, oncology specialists will encounter older patients with cancer more frequently. Cancer incidence increases with age and most cancer patients are over the age of 65. For breast cancer, the incidence is expected to rise in the coming decades, partly due to a longer life expectancy and socioeconomic transitions (1). One-third of patients with breast cancer are 70 years or older at the time of diagnosis (2). The care of these older patients can be complex, due to comorbidity and frailty, leading to a higher risk of adverse events and of unwanted treatment outcomes. Frail older patients are underrepresented in clinical trials and when they are represented, relevant endpoints are often not assessed (3-6). Advances in breast cancer screening and treatment have improved the prognosis of patients with breast cancer over time. However, despite an often favourable molecular and biological phenotype of breast cancer, older patients have worse survival rates than their younger counterparts. This may be attributable to more advanced disease at diagnosis, a higher burden of comorbid conditions and frailty. Also, under- and overtreatment may play a role; despite guidelines recommending treatment irrespective of age, older patients with breast cancer are more likely to receive less intensive treatment (2,7).
Since geriatricians are specialized in assessing and treating older adults with multimorbidity and frailty, a collaboration between oncology specialists and geriatricians will often be valuable. Geriatric oncology focusses on assessment of older patients with cancer, aimed at prognostication, supporting decision-making and on provision of non-oncological advice to prevent complications and enhance recovery. Geriatric oncology is a rapidly growing field, with an increasing number of publications since the beginning of this century (Figure 1). In this review, we describe the available evidence on geriatric assessment to guide oncological treatment and describe possibilities to ensure collaboration between oncology specialists and geriatricians in the care for older cancer patients.
The importance of frailty
There can be large heterogeneity between patients of the same age and gender with regards to comorbidity, level of dependence, cognitive function and physical reserves. Chronological age is an insuffcient marker of these differences in ‘biological age’ and therefore of fitness for treatment. Older patients are at risk of undertreatment (i.e., refraining from a potential curative treatment based on the patients age) as well as overtreatment (starting a treatment with a high risk of adverse outcomes) (8). Frailty, a loss of reserves that leads to increased risk of adverse events, is a more suitable measure of biological age and a powerful predictor of adverse outcomes (9). Frailty results from a decline in multiple physiological systems over the course of a life, leading to an accumulation of deficits (10). Patients with frailty have a reduced ability to cope with stressors such as surgery and chemotherapy (11,12). Frailty is prevalent in older patients with cancer with more than half being frail or pre-frail (9). Even though there is a relation between frailty, comorbidity and disability, they are also distinct entities and patients can be frail despite not having relevant comorbidies or disabilities (13). Determining frailty based on clinical judgment of a healthcare professional (eyeballing), has been shown to be insufficient (14,15). Geriatric assessment can reveal frailty and further aid in prognostication and guide treatment decision-making.
What does a geriatrician do?
Geriatricians are medical doctors who work in a multidisciplinary fashion in order to optimize health, function, and quality of life for older complex patients. Thanks to advances in life expectancy following effective treatment for a number of physical illnesses due to procedures and medications, many older individuals accumulate a long list of comorbidities and polypharmacy. Thus, being a specialist in only one organ or one disease may preclude the holistic approach needed for the care of an older patient. Furthermore, functional decline and frailty increases with advancing age. Adding on cognitive impairment or dementia, atypical presentation of disease and ethical issues towards the end of life—and it is easy to understand why a geriatrician needs to work in multidisciplinary teams consisting of geriatric nurses, physical therapists, occupational therapists, social workers, and dieticians. In order to assess the patient comprehensively, geriatric assessment is necessary. Models of delivering geriatric medicine vary between countries and health care systems—examples are home visits, acute hospital wards, outpatient clinics and rehabilitation wards, but all models share the goal of optimizing health, function, and quality of life in older complex patients. In geriatric oncology, most of the studies have incorporated management as a consultation service to surgeons and oncologists.
Geriatric assessment
Geriatric assessment is a systematic assessment of multiple areas in an individual patient, giving insight into the somatic, psychological, functional and social domains, identifying strengths and deficits. Comorbidity, polypharmacy, (mal) nutrition, cognitive status and psychological problems (such as depression and anxiety), dependencies in activities of daily living (ADL), such as dressing and grooming, and instrumental activities of daily living ( iADL), such as cooking a meal and handling finances, falls, mobility, social status and living situation are assessed during a geriatric assessment (16,17) (Table 1).
Table 1
| Domain | Subdomain | Examples of assessment tools/questions |
|---|---|---|
| Somatic | Comorbidity | Charlson Comorbidity Index (CCI), Cumulative Illness Rating Scale for Geriatrics (CIRS-G) (18,19) |
| Polypharmacy | Number of medications, STOPP/START criteria (20,21) | |
| Nutrition | Weight loss and BMI | |
| Mini Nutritional Assessment (MNA) (short version) | ||
| Patient-generated Subjective Global Assessment (PG-SGA) (22) | ||
| Psychological | Cognition | Mini Mental State Examination (MMSE) |
| Montreal Cognitive Assessment (MoCA), Blessed Orientation Memory Concentration test/6-item Cognitive Impairment Test (BOMC/6-CIT) | ||
| Clock drawing test | ||
| Mini-COG (23-25) | ||
| Mood (depression, anxiety) | Geriatric Depression Scale (GDS) | |
| Patient Health Questionnaire (PHQ-2, PHQ-4, PHQ-9) Hospital Anxiety depression Scale (HADS) (26-28) | ||
| Intoxications | Smoking, alcohol intake | |
| Social | Marital status | Partner, children |
| Living situation | Dependent or independent living, living alone | |
| Formal and informal care | ||
| Functional | Physical performance | Timed-Up and Go Test (TUG) |
| 4m Gait Speed | ||
| Short Physical performance battery (SPPB) | ||
| Gripth strength (29) | ||
| Falls | Number of falls previous six moths | |
| Activities of daily living (ADLs) | Katz index of Independence in Activities of Daily Living (30) | |
| Instrumental activities of daily living (iADLs) | Lawton scale for instrumental Activities of Daily Living | |
| Sensory impairments | Hearing impairment or visual problems |
Geriatric assessment can refer to a Comprehensive Geriatric Assessment (CGA), an (abbreviated) geriatric assessment or to geriatric screening. These terms are often used interchangeably in the literature, however, there is a difference. A CGA can be defined as ‘a multi-dimensional, interdisciplinary, diagnostic process to identify care needs, plan care, and improve outcomes of frail older people’ (31). A CGA is aimed at diagnostics, prognostication and interventions, and is usually performed by a geriatrician in a multidisciplinary setting. A geriatric assessment is a shorter assessment of geriatric domains, mostly aimed at providing a diagnosis, and can be performed by trained healthcare professionals. Geriatric screening refers to the use of a single short screening tool to identify patients that are likely to benefit form a more extensive geriatric assessment (32).
In oncology, geriatric assessment is used to assess the level of frailty and thereby predict adverse events, such as complications, toxicity and mortality. It has been shown that incorporating geriatric assessment in decision-making for older patients with cancer, leads to an adjustment of cancer treatment in about one in four patients (33). Optimization (e.g., prehabilitation) and preventive measures (e.g., delirium risk and risk of falls) can be advised based on geriatric assessment. Providing geriatric assessment information to oncologists has been shown to reduce toxicity, with similar survival (34). Geriatric assessment has also been shown to enhance communication between the patient and the healthcare professional (35). For older patients receiving chemotherapy, prediction tools incorporating geriatric parameters, such as the CARG (Cancer and Aging Research Group) and CRASH (Chemotherapy Risk Assessment Scale) scores, have been developed to predict the risk of toxicity (36,37). These tools incorporate disease related items and geriatric variables and are well validated (38).
Implementation of geriatric assessment in oncology clinical practice
Geriatric assessment in oncology has been recommended by the International Society of Geriatric Oncology (SIOG) guidelines, and the American Society of Clinical Oncology (ASCO) guideline recommends GA for all patients 65 years and older receiving chemotherapy (16,17).
There are different ways to incorporate geriatrics into the management of older patients with cancer, depending on the population and on local health resources (11). The first option is to refer all older patients to a geriatrician for CGA, based on calendar age. CGA typically requires expertise from a geriatrician, is performed in a multidisciplinary setting, and takes time to complete. With the increasing number of older patients with cancer, and the limited number of geriatricians, this may not be feasible, especially in area’s with limited healthcare resources and may also not be preferable from a patient perspective. CGA should best be reserved for patients who are most likely to benefit from an extensive assessment. The second option is to select patients based on an abbreviated geriatric assessment which can be performed by other healthcare professionals, such as an oncology nurse, in close collaboration with a geriatrician. Such an assessment consists of a semi-structured interview and/or combination of geriatric screening instruments in order to assess different geriatric domains. Self-report by patient and caregivers has also been used (34). Patients who need a more extensive assessment, based on the findings of the abbreviated GA, can be referred to a geriatrician for CGA, but the number of patients in need of referral has been shown to be low (13%) (39). The third option is to use a short frailty screening test to identify patients who would benefit from a CGA, such as the Geriatric 8 (G8) and Vulnerable Elders Survey 13 (VES-13). However, these tests have moderate sensitivity and specificity (40). A recent study in 177 patients with primary localized breast cancer aged ≥70 years identified 52% of patients as frail using the G8 (41). This option may therefore seem easier at first glance, but may be more time consuming due to a higher referral rate.
Assessment of patient goals and preferences
Assessment of goals and preferences is considered an important step in shared decision-making. This enables the alignment of treatment options to goals and preferences, thereby tailoring treatment to the individual patient. With aging, goals and preferences can change. Quality of life, physical and cognitive function and remaining independent are often more highly valued by older patients than extending life (42-44). Intensive treatment regimes comprise trade-offs; e.g., breast cancer surgery or chemotherapy that is aimed at improving survival, can at the same time result in prolonged recovery or even loss of independence for frail older patients. It is important to discuss these trade-offs beforehand and gain insight into the patient’s preferences and priorities during shared decision-making. Questions such as: “What matters most to you in your life?” can give an insight into what is important and provides an opportunity to discuss how a certain treatment might influence this priority. Asking a patient “what do you expect from this treatment?” can give insight into the patient’s understanding of the decision at hand and guide a discussion about trade-offs. Decision aids, such as the Outcome Prioritization Tool, can support a conversation about goals and preferences (45). Without discussing goals and preferences explicitly, healthcare professionals often have poor knowledge about what matters most to their patient (46-48).
Multidisciplinary decision-making for older patients with cancer
In many countries, decision-making for patients with cancer takes place in multidisciplinary teams (MDTs). Currently, these teams focus on disease-specific information, and little information is provided regarding patient-specific aspects, such as level of frailty, physical and cognitive functioning and patient preferences. Observations of oncological MDTs have shown that information about the patient’s circumstances and preferences was rarely discussed during these meetings and even information on comorbidities was often not reviewed (49-51).
For patient-centered decision-making it is important to not only consider disease-specific information, but to also take patient-specific information and the time perspective into account (Figure 2). Patient-specific information can guide shared decision-making by aligning treatment options to the level of frailty and the context (“who is this patient”) and to the goals and preferences (“what does this patient want”) of the individual patient.
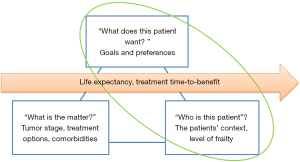
Older patients have a limited remaining life expectancy, but this may differ between patients of the same age and gender due to comorbidity, lifestyle and frailty (52). For instance, older patients with breast cancer and dementia have a higher mortality than patients without dementia (53). An estimation of the patients remaining life expectancy allows for taking the time perspective into account in the decision-making, thereby preventing both over- and undertreatment. If the time-to-benefit of the treatment option (the time between starting a treatment and the moment that the patient experiences benefit) is longer than the life expectancy, the patient will not experience the beneficial effects. Clinicians have been shown to be poor in predicting a patient’s life expectancy, and tend to overestimate (54). A more reliable estimation can be made using prognostic models such as The Lee-Schonberg prognostic model that predicts 5 and 10 year life expectancy (55,56). Prediction models can be found on the website eprognosis (https://eprognosis.ucsf.edu/).
To tailor decision-making to the individual patient, disease-specific and patient-specific information and life expectancy can be weighed in a stepwise fashion, using the following questions (39):
- What is the indication for the treatment, the goal of the treatment and what are the treatment alternatives;
- What are the expected outcomes of the different treatment options (including palliative treatment or wait and see), based on the treatment intensity and the patient’s frailty;
- What is the life expectancy of the patient and what is the time to benefit of the different treatment options (including palliative treatment and wait and see);
- What are the goals and preferences of the patient and how do the expected outcomes of different treatment options align to these goals and preferences.
Patient involvement in treatment decision-making
For optimal shared decision-making, patient involvement is important. However, decision-making in collaboration with older patients with cancer can be challenging (57). Furthermore, cognitive decline and limited health literacy can interfere with decision-making capacity, and older patients often have other goals and preferences regarding outcomes of cancer treatment compared to younger patients. This complicates decision-making and calls for a tailored and patient-centered approach. Treatment decision in older patients are more often preference sensitive. Eliciting the patient’s goals and preferences is therefore an important step in the decision-making process. With increasing age, the incidence of cognitive impairment or dementia rises. Patients with cognitive impairment might be less able to oversee different treatment options. This is important to assess and to take into account during decision making, since cognitive impairment is often undiagnosed and can be easily missed (58). Caregivers can play an important role in the decision-making process and it is important to involve them, since they may have knowledge of the patients preferences and values.
Non-oncological advice and geriatric co-management
Since geriatric assessment uncovers problems in geriatric domains, it also provides an opportunity for individualized patient management before, during, and after oncological treatment. A systematic review showed that based on geriatric assessment, non-oncological advice was provided for 72% of patients (33). The most commonly provided advise was interventions regarding social, nutritional and medication issues. In order to optimize the patient’s health status before treatment, prehabilitation (e.g., optimization of nutritional status, strength/endurance exercises and coaching) can be advised for selected patients (59,60). Consulting a psychiatrist may be appropriate for patients with depression, anxiety or psychiatric illness (61). During hospital admission, preventive measures can be provided for patients who are at a high risk for complications, such as delirium or falls. Geriatric co-management might be appropriate for these high-risk patients. Following treatment, rehabilitation can enhance recovery.
Effect of implementing geriatric assessment in oncology on treatment outcomes
The association of geriatric assessment with adverse outcomes of cancer treatment has been well established in the literature (62). Studies revealing improved outcomes based on integrating geriatric assessment in oncology are still limited;however, a recent article gives an overview of 20 publications (full papers and abstracts) of the effect of geriatric assessment and geriatric management on outcomes (63). Toxicity and treatment completion were the most frequently reported outcomes. Only four studies presented patient-centered outcomes, and 11 articles were only available as an abstract. Most evidence is available for chemotherapy, some for surgery, while studies on the outcomes of geriatric assessment for radiation therapy are still lacking. Recently two large randomized clinical trials investigating the value of GA and GA-driven management recommendations in older patients with cancer were published that provide strong support for the implementation of GA in clinical practice . The GAP70+ study included 718 patients of 70 years and older with incurable solid tumors or lymphoma and at least one impaired geriatric domain eligible to receive palliative chemotherapy (64). These patients were randomized to an intervention in which a summary of the results of a geriatric assessment and management recommendations were provided to the medical oncologist, versus care as usual. The intervention group experienced significantly less grade 3–5 toxicity (51% versus 71% in the care as usual group, P=0.0001) using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, version 4.0). The intervention group also experienced fewer falls and had more medications discontinued. No differences in survival were observed. The GAIN study randomized 613 patients of 65 years and older with different solid malignancies who started chemotherapy (65). All patients received a geriatric assessment before starting treatment, but in the intervention group this was discussed in a geriatrics-trained multidisciplinary team and interventions, based on the assessment, were implemented. In the care as usual group the results of the GA were sent to the oncologists for self-review. The primary outcome was grade 3 or higher chemotherapy toxicity using NCI CTCAE v4.0 criteria, with a significantly lower rate of toxicity in the intervention arm (50.5% versus 60.6%, P=0.02). There was a significant increase in advance directives completion in the intervention group. There were no differences in the number of visits to the emergency department, hospitalizations or overall survival between the groups.
Geriatric assessment in the management of older patients with breast cancer
A recent joint guideline from The European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG) recommends assessment of frailty, consideration of life expectancy, and assessment of patient preferences in treatment decision-making for all patients with breast cancer aged ≥70 years (66). These recommendations are supported by a recent systematic review that summarized the evidence on predictive factors for disease-related outcomes, survival, toxicity and patient-reported outcomes in older patients with early and advanced breast cancer (67). Besides age and disease-related characteristics, the review found that multiple variables from geriatric assessment predicted all outcomes. In 19 out of 26 studies looking at patient-reported outcomes such as physical functioning, cognitive functioning, life satisfaction or mental health, geriatric measures were found to be predictors.
Studies on the value of CGA in treatment decision-making or as a prognostic tool in breast cancer included heterogeneous patient groups and used a variety of screening instruments to assess CGA domains (7). As a result, it is unclear which frailty screening tools are most appropriate, and which breast cancer patients are most likely to benefit from geriatric assessment. Nevertheless, The 2021 EUSOMA-SIOG recommendations state that the use of a frailty screening tool should be considered the minimum starting point for any treatment decision in older patients with breast cancer (66).
Recently a study was published that described the development and validation of a new prognostic tool, the PORTRET tool (68). This is a prediction tool for 5-year recurrence and mortality for patients with early invasive breast cancer of 65 years and older and was developed because other prediction tools (Adjuvant! Online and PREDICT), were insufficient in predicting outcomes in the oldest patients and patients with multimorbidity. The PORTRET tool combines disease and patient characteristics and predicts 5-year recurrence and mortality in older patients with breast cancer, with an area under the curve of 0.75–0.76. However, patient-reported outcomes were not incorporated in this tool.
Conclusions
Geriatric assessment and geriatric co-management can guide treatment decision-making and provide advice for pre- and post-treatment optimization for older patients with breast cancer. Geriatric assessment is superior to clinical judgement in predicting adverse treatment outcomes. The evidence of the added value of geriatric assessment in oncology is rapidly increasing. Incorporation of geriatric assessment in oncology care is advocated by different guidelines; recently specifically for older patients with breast cancer as well. Frailty screening is advised for all patients prior to treatment, in addition to assessment of patients preferences and life expectancy. This can be implemented in different ways depending on local healthcare resources. Involving a geriatrician in your oncological team can support the implementation of geriatric assessment for older cancer patients and provides an opportunity to share expertise. More large studies on outcomes are needed especially for surgery and radiation therapy, where evidence on outcomes is still scarce. It is also important to enhance our knowledge of the treatment outcomes that matter to our older patients. For this, we need to expand our focus from traditional outcomes, such as complications and survival, to also include quality of life, and cognitive and physical functioning.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kwok-Leung Cheung) for the series “Diagnosis and Treatment on Primary Breast Cancer in Older Women” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-119/coif). The series “Diagnosis and Treatment on Primary Breast Cancer in Older Women” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lima SM, Kehm RD, Terry MB. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine 2021;38:100985. [Crossref] [PubMed]
- Bagegni NA, Peterson LL. Age-related disparities in older women with breast cancer. Adv Cancer Res 2020;146:23-56. [Crossref] [PubMed]
- Marosi C, Köller M. Challenge of cancer in the elderly. ESMO Open 2016;1:e000020. [Crossref] [PubMed]
- de Glas NA, Hamaker ME, Kiderlen M, et al. Choosing relevant endpoints for older breast cancer patients in clinical trials: an overview of all current clinical trials on breast cancer treatment. Breast Cancer Res Treat 2014;146:591-7. [Crossref] [PubMed]
- Hamaker ME, Stauder R, van Munster BC. On-going clinical trials for elderly patients with a hematological malignancy: are we addressing the right end points? Ann Oncol 2014;25:675-81. [Crossref] [PubMed]
- Vodicka E, Kim K, Devine EB, et al. Inclusion of patient-reported outcome measures in registered clinical trials: Evidence from ClinicalTrials.gov (2007-2013). Contemp Clin Trials 2015;43:1-9. [Crossref] [PubMed]
- Jauhari Y, Gannon MR, Dodwell D, et al. Addressing frailty in patients with breast cancer: A review of the literature. Eur J Surg Oncol 2020;46:24-32. [Crossref] [PubMed]
- DuMontier C, Loh KP, Bain PA, et al. Defining Undertreatment and Overtreatment in Older Adults With Cancer: A Scoping Literature Review. J Clin Oncol 2020;38:2558-69. [Crossref] [PubMed]
- Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2015;26:1091-101. [Crossref] [PubMed]
- Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752-62. [Crossref] [PubMed]
- Puts M, Soo WK, Szumacher E, et al. Methods for frailty screening and geriatric assessment in older adults with cancer. Curr Opin Support Palliat Care 2021;15:16-22. [Crossref] [PubMed]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. [Crossref] [PubMed]
- Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255-63. [Crossref] [PubMed]
- Kirkhus L, Šaltytė Benth J, Rostoft S, et al. Geriatric assessment is superior to oncologists' clinical judgement in identifying frailty. Br J Cancer 2017;117:470-7. [Crossref] [PubMed]
- van Walree IC, Scheepers ERM, van den Bos F, et al. Clinical judgment versus geriatric assessment for frailty in older patients with cancer. J Geriatr Oncol 2020;11:1138-44. [Crossref] [PubMed]
- Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595-603. [Crossref] [PubMed]
- Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018;36:2326-47. [Crossref] [PubMed]
- Marventano S, Grosso G, Mistretta A, et al. Evaluation of four comorbidity indices and Charlson comorbidity index adjustment for colorectal cancer patients. Int J Colorectal Dis 2014;29:1159-69. [Crossref] [PubMed]
- Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 1992;41:237-48. [Crossref] [PubMed]
- Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr 2017;17:230. [Crossref] [PubMed]
- O'Mahony D. STOPP/START criteria for potentially inappropriate medications/potential prescribing omissions in older people: origin and progress. Expert Rev Clin Pharmacol 2020;13:15-22. [Crossref] [PubMed]
- Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779-85. [Crossref] [PubMed]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. [Crossref] [PubMed]
- Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry 2007;52:329-32. [Crossref] [PubMed]
- Creavin ST, Wisniewski S, Noel-Storr AH, et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev 2016;CD011145. [Crossref] [PubMed]
- Annunziata MA, Muzzatti B, Bidoli E, et al. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support Care Cancer 2020;28:3921-6. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284-92. [Crossref] [PubMed]
- Pocklington C, Gilbody S, Manea L, et al. The diagnostic accuracy of brief versions of the Geriatric Depression Scale: a systematic review and meta-analysis. Int J Geriatr Psychiatry 2016;31:837-57. [Crossref] [PubMed]
- Verweij NM, Schiphorst AH, Pronk A, et al. Physical performance measures for predicting outcome in cancer patients: a systematic review. Acta Oncol 2016;55:1386-91. [Crossref] [PubMed]
- Couderc AL, Suchon P, Saliba-Serre B, et al. Functional status in older patients with cancer. J Geriatr Oncol 2022;13:40-5. [Crossref] [PubMed]
- Rubenstein LZ, Joseph T. Freeman award lecture: comprehensive geriatric assessment: from miracle to reality. J Gerontol A Biol Sci Med Sci 2004;59:473-7. [Crossref] [PubMed]
- Puts MTE, Alibhai SMH. Fighting back against the dilution of the Comprehensive Geriatric Assessment. J Geriatr Oncol 2018;9:3-5. [Crossref] [PubMed]
- Hamaker ME, Te Molder M, Thielen N, et al. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients - A systematic review. J Geriatr Oncol 2018;9:430-40. [Crossref] [PubMed]
- Mohile SG, Mohamed MR, Culakova E, et al. A geriatric assessment (GA) intervention to reduce treatment toxicity in older patients with advanced cancer: A University of Rochester Cancer Center NCI community oncology research program cluster randomized clinical trial (CRCT). J Clin Oncol 2021;38:12009. [Crossref]
- Mohile SG, Epstein RM, Hurria A, et al. Communication With Older Patients With Cancer Using Geriatric Assessment: A Cluster-Randomized Clinical Trial From the National Cancer Institute Community Oncology Research Program. JAMA Oncol 2020;6:196-204. [Crossref] [PubMed]
- Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377-86. [Crossref] [PubMed]
- Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457-65. [Crossref] [PubMed]
- Ostwal V, Ramaswamy A, Bhargava P, et al. Cancer Aging Research Group (CARG) score in older adults undergoing curative intent chemotherapy: a prospective cohort study. BMJ Open 2021;11:e047376. [Crossref] [PubMed]
- Festen S, Kok M, Hopstaken JS, et al. How to incorporate geriatric assessment in clinical decision-making for older patients with cancer. An implementation study. J Geriatr Oncol 2019;10:951-9. [Crossref] [PubMed]
- Hamaker ME, Jonker JM, de Rooij SE, et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol 2012;13:e437-44. [Crossref] [PubMed]
- Scheepers ERM, van Huis-Tanja LH, Emmelot-Vonk MH, et al. Study objectives in clinical trials in older patients with solid malignancies: do we measure what matters? Qual Life Res 2021;30:1833-9. [Crossref] [PubMed]
- Fried TR. Shared Decision Making--Finding the Sweet Spot. N Engl J Med 2016;374:104-6. [Crossref] [PubMed]
- Fried TR, Van Ness PH, Byers AL, et al. Changes in preferences for life-sustaining treatment among older persons with advanced illness. J Gen Intern Med 2007;22:495-501. [Crossref] [PubMed]
- Naik AD, Martin LA, Moye J, et al. Health Values and Treatment Goals of Older, Multimorbid Adults Facing Life-Threatening Illness. J Am Geriatr Soc 2016;64:625-31. [Crossref] [PubMed]
- Stegmann ME, Festen S, Brandenbarg D, et al. Using the Outcome Prioritization Tool (OPT) to assess the preferences of older patients in clinical decision-making: A review. Maturitas 2019;128:49-52. [Crossref] [PubMed]
- Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients' preferences matter. BMJ 2012;345:e6572. [Crossref] [PubMed]
- Festen S, Stegmann ME, Prins A, et al. How well do healthcare professionals know of the priorities of their older patients regarding treatment outcomes? Patient Educ Couns 2021;104:2358-63. [Crossref] [PubMed]
- Montgomery AA, Fahey T. How do patients' treatment preferences compare with those of clinicians? Qual Health Care 2001;10:i39-43. [Crossref] [PubMed]
- Bolle S, Smets EMA, Hamaker ME, et al. Medical decision making for older patients during multidisciplinary oncology team meetings. J Geriatr Oncol 2019;10:74-83. [Crossref] [PubMed]
- Holden CA, Poprawski D, Singhal N, et al. A systematic scoping review of determinants of multidisciplinary cancer team access and decision-making in the management of older patients diagnosed with colorectal cancer. J Geriatr Oncol 2020;11:909-16. [Crossref] [PubMed]
- Festen S, Nijmeijer H, van Leeuwen BL, et al. Multidisciplinary decision-making in older patients with cancer, does it differ from younger patients? Eur J Surg Oncol 2021;47:2682-8. [Crossref] [PubMed]
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA 2001;285:2750-6. [Crossref] [PubMed]
- Raji MA, Kuo YF, Freeman JL, et al. Effect of a dementia diagnosis on survival of older patients after a diagnosis of breast, colon, or prostate cancer: implications for cancer care. Arch Intern Med 2008;168:2033-40. [Crossref] [PubMed]
- Krishnan M, Temel JS, Wright AA, et al. Predicting life expectancy in patients with advanced incurable cancer: a review. J Support Oncol 2013;11:68-74. [Crossref] [PubMed]
- Lee SJ, Lindquist K, Segal MR, et al. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 2006;295:801-8. [Crossref] [PubMed]
- Schonberg MA, Davis RB, McCarthy EP, et al. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med 2009;24:1115-22. [Crossref] [PubMed]
- Rostoft S, van den Bos F, Pedersen R, et al. Shared decision-making in older patients with cancer - What does the patient want? J Geriatr Oncol 2021;12:339-42. [Crossref] [PubMed]
- Amjad H, Roth DL, Sheehan OC, et al. Underdiagnosis of Dementia: an Observational Study of Patterns in Diagnosis and Awareness in US Older Adults. J Gen Intern Med 2018;33:1131-8. [Crossref] [PubMed]
- Hamaker ME, Oosterlaan F, van Huis LH, et al. Nutritional status and interventions for patients with cancer - A systematic review. J Geriatr Oncol 2021;12:6-21. [Crossref] [PubMed]
- Souwer ETD, Bastiaannet E, de Bruijn S, et al. Comprehensive multidisciplinary care program for elderly colorectal cancer patients: "From prehabilitation to independence". Eur J Surg Oncol 2018;44:1894-900. [Crossref] [PubMed]
- Saracino RM, Nelson CJ. Identification and treatment of depressive disorders in older adults with cancer. J Geriatr Oncol 2019;10:680-4. [Crossref] [PubMed]
- Hamaker ME, Wildes TM, Rostoft S. Time to Stop Saying Geriatric Assessment Is Too Time Consuming. J Clin Oncol 2017;35:2871-4. [Crossref] [PubMed]
- Rostoft S, O'Donovan A, Soubeyran P, et al. Geriatric Assessment and Management in Cancer. J Clin Oncol 2021;39:2058-67. [Crossref] [PubMed]
- Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet 2021;398:1894-904. [Crossref] [PubMed]
- Li D, Sun CL, Kim H, et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults With Cancer: A Randomized Clinical Trial. JAMA Oncol 2021;7:e214158. [Crossref] [PubMed]
- Biganzoli L, Battisti NML, Wildiers H, et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol 2021;22:e327-40. [Crossref] [PubMed]
- van der Plas-Krijgsman WG, de Boer AZ, de Jong P, et al. Predicting disease-related and patient-reported outcomes in older patients with breast cancer - a systematic review. J Geriatr Oncol 2021;12:696-704. [Crossref] [PubMed]
- van der Plas-Krijgsman WG. GD, Putter H, Steyerberg E, et al. Development and validation of the PORTRET tool to predict recurrence, overall survivalk, and other-cause mortality in older patients with breast cancer in the Netherlands: a population-based study. The Lancet Healthy Longevity 2021;2:e704. [Crossref] [PubMed]
Cite this article as: Festen S, de Graeff P, Rostoft S. The role of the geriatrician in the care of older patients with breast cancer: a review. Ann Breast Surg 2023;7:29.