Sensory reinnervation after mastectomy with implant-based reconstruction
Post-mastectomy sensation following traditional approaches
Loss of breast sensation after mastectomy has gained more attention recently, particularly as it has been shown to be associated with a negative psychosocial impact for patients and certainly contributes to the decreased breast-specific sensuality and quality of life outcomes seen after mastectomy (1,2). Although there have been remarkable overall advancements in oncologic as well as reconstructive treatment since Halstead first described the radical mastectomy in the late 19th century (3,4), optimal sensation following mastectomy and breast reconstruction still remains one of the final hurdles in the field of breast cancer treatment.
Advances in mastectomy techniques such as preservation of the entire skin envelope through nipple-sparing mastectomy have certainly improved aesthetic outcomes (5), though hoped for improvements in sensation haven’t been seen to the same degree. A number of studies have looked at sensation outcomes following nipple-sparing mastectomy and found overall relatively low rates of sensation preservation. Dosset et al. found measurable sensation in the nipple-areolar complex (NAC) in only 28% of patients undergoing nipple-sparing mastectomy at least a year following their reconstruction with either expanders/implants or autologous tissue (6). Moreover, the degree of measured sensation that was present was quite poor. Rodriguez-Unda et al. similarly reported decreased post-operative breast sensation following reconstruction in both skin-sparing mastectomies and nipple-sparing mastectomy patients (7). Yet another study from Sweden reported similar significantly impaired breast sensibility when comparing patients undergoing prophylactic, mastectomy with free nipple grafting (8). The same study further demonstrated that ability to experience sexual sensation was lost, perhaps explaining a primary reluctance of many women to undergo risk-reduction surgery.
Having immediate reconstruction at the time of mastectomy can certainly optimize aesthetic results, but also could potentially have been thought to improve sensation as well. However, results with sensation following mastectomy with immediate reconstruction have also been sub-optimal. Yueh et al. looked at 17 breasts in 10 patients who underwent nipple-sparing mastectomy with immediate reconstruction. While there was some measurable sensation in the NAC in a number of patients post-operatively, the degree of sensation was quite low (9). In a larger cohort, Djohan et al. reported that a majority of patients described fair or poor NAC sensation after nipple-sparing mastectomy and immediate reconstruction, even with a mean follow-up of greater than four years (10). Patient-reported outcomes from this study showed that this loss of sensation was the part of the patients’ results that they would most like to change. Finally, data from Peled et al. in patients undergoing nipple-sparing mastectomy with immediate expander-implant reconstruction demonstrated that only 3% of patients were “very satisfied” and 21% of patients “somewhat satisfied” with their NAC sensation (11).
Relevant breast anatomy for facilitating sensory reinnervation
Ongoing study of the sensory innervation to the breast has helped to better define the anatomy and allow for targeted nerve preservation and reinnervation. Specifically, sensation to the breast skin envelope derives predominantly from the 1st–6th medial intercostal and 2nd–7th lateral intercostal nerves (12). Most papers also describe the sensory innervation to the NAC itself primarily from the medial and lateral superficial branches of the 3rd–5th intercostal nerves (13,14). Understanding of this anatomic landscape has allowed a number of authors to suggest modifications to established oncologic and reconstructive breast surgical procedures to help minimize sensory loss post-operatively.
Schulz et al. described limiting dissection within the inferolateral quadrant of the breast during reduction mammaplasty to optimize NAC sensation post-surgery (15). The lateral intercostal nerves typically have both superficial and deep branches, with the former often coursing in the subcutaneous tissues of the lateral skin flap and the former taking intra-parenchymal routes to reach the sub-areolar region and innervate the nipple-areolar complex. Knackstedt et al. defined the anatomy of the lateral intercostal nerve as emerging within 2 cm of the lateral border of the pectoralis minor muscle and predictably travelling under the adjacent vessels (16). They opined that recognition of this anatomy during mastectomy could aid the operative surgeon in identifying and ideally preserving the relevant nerve(s) to optimize post-operative sensation. Although consideration of mastectomy skin flap thickness has been described as it relates to reconstructive and oncologic outcomes (17,18), the correlation between skin flap thickness and sensory nerve preservation has not been well-studied.
In addition to the sensory anatomy of the breast, a better understanding of feasible donor nerve anatomy in those for whom an autologous reconstruction is contemplated has also developed. With DIEP reconstruction, several authors have described the donor nerve(s) for a possible sensate flap utilizing the 10th–12nd intercostal nerves in the upper abdomen (19). Momeni et al. additionally described limiting donor nerve harvesting to the more distal, sensory portion of those caudal intercostal nerves (20).
Advances in post-mastectomy sensory preservation and nerve reconstruction
Given the low reported rates of sensation preservation following traditional mastectomy as described above, surgeons have begun to develop better techniques to help preserve and restore sensation. Until recently, the approaches have solely involved restoring sensation with autologous reconstruction, though a few newer studies have shown promising outcomes with implant-based reconstruction.
Some of the earliest studies of breast neurotization were done with neurotized TRAM flaps, which showed potential benefit with regards to improving sensation, but a definitive improvement from neurotization as compared to spontaneous sensory recovery was unclear (21). Sensory restoration with autologous reconstruction has evolved over time and has been reported in several studies using neurotized DIEP or other perforator flaps, primarily to improve sensation with delayed reconstruction using direct neurotization (22,23). Some larger studies have shown results with both immediate and delayed reconstruction using both nerve conduits and direct innervation of flap skin islands, with significant improvement in recovery of sensation when compared to controls (24,25). Emphasis on standardization of technique and outcomes measurements has been seen in recent years to help optimize outcomes (26) including the creation of a multi-site prospectively collected registry that has been shown to have promising early results (27).
To date, the literature on sensation reinnervation with implant reconstruction is very limited given the technique has only recently been adopted and in a small number of centers. The two published studies specifically addressing outcomes after neurotization with implant-based reconstruction were both done with immediate neurotization done at the time of mastectomy. Our previously reported work (28) presented proof of concept and efficacy data on intercostal nerve preservation as well as nipple-areolar complex neurotization done at the time of nipple-sparing mastectomy in the setting of immediate, pre-pectoral, direct-to-implant reconstruction. Results from 32 mastectomies in 17 patients showed preservation of sensation as measured with two-point discrimination in 88% of cases. 94% of patients had gross sensation to light touch throughout all 4 quadrants of their mastectomy skin. Although overall outcomes showed good return of sensation in the patient cohort, quantitative outcomes measures specifically looking at skin flap sensation were limited, as was follow-up time and patient-reported outcomes data. Another recent study from Djohan et al. (29) demonstrated similar results in patients undergoing nipple-areolar complex neurotization at the time of implant-based reconstruction. Their study presented sensory outcomes from 15 mastectomies in 8 patients using a pressure-specified sensory device to assess sensation. They found overall improvements in mastectomy skin and nipple-areolar complex sensation over time, though acknowledged that their follow-up was limited and that further recovery could be anticipated with additional follow-up. Additionally, their study did not include any patient-reported data and their cohort size was small and heterogeneous.
Outcome metrics to gauge breast sensation have also seen improvements with time. Older methods of assessing breast sensibility have included gross light touch, pin-prick, perception of pain to electric stimulation, sensitivity to vibration, thermal sensation and most commonly, Semmes-Weinstein monofilament testing (30,31). More recently, use of cutaneous pressure thresholds (pressure-specified sensory device measurements) have been utilized to measure post-surgical breast sensation with several advantages (29,32). First, the results obtained are linear as opposed to logarithmic (for Semmes-Weinstein monofilaments) thus making them more amenable to statistical analysis. Second, cutaneous pressure thresholds are able to be tested for both static and moving stimuli allowing evaluation of both Merkel cell-neurite/Raffini complexes as well as Meissner/Pacinian corpuscles, respectively. Calibration is also easily performed making the results more reliable and accurate (33). Furthermore, with the advent of the BREAST-Q, validated and reliable PROMs are readily accessible to study the effects of mastectomy and reconstruction, including sensation (34).
Practice experience with sensation-preserving mastectomy and implant reconstruction
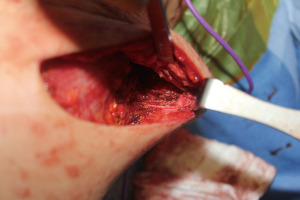
We began performing sensation-preserving mastectomies with immediate implant-based reconstruction in our practice in February 2018 and have now done over 200 of these procedures. The technique, which has been previously described (28), involves both intercostal nerve preservation and nerve grafting from intercostal nerves to subareolar nerves. Briefly, during mastectomy (almost exclusively nipple-sparing mastectomy in our practice), care is taken during the lateral dissection to preserve the lateral, superficial branches of the 3rd, 4th or 5th intercostal nerves whenever possible at the thoracic cage. Preservation is done when considered oncologically safe as defined by favorable anatomy with the nerves running within the subcutaneous tissue and not through the breast parenchyma itself (Figure 1). If identified nerves are found to be running through the breast parenchyma, they are carefully dissected out to length within the parenchyma until no longer oncologically safe, at which point the nerves are sharply transected. In order to optimize oncologic safety, no glandular breast tissue is intentionally left behind during the mastectomy for the sake of nerve preservation.
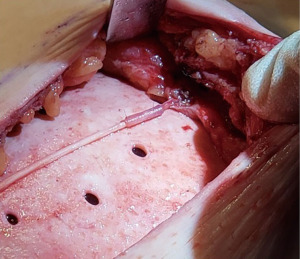
For those nerves that are transected, nerve reconstruction is performed using an Avance nerve allograft (Axogen, Jacksonville, FL, USA) coapted from the transected lateral intercostal nerve to an identified subareolar nerve (Figures 2,3). The nerve reconstruction is done after pre-pectoral implant reconstruction with anterior, acellular dermal matrix (ADM) coverage with the reconstructed nerve lying in the plane between the ADM and subcutaneous tissue. In addition, connector-assisted repairs are performed to reduce tension at the nerve coaptation site, to reduce suture burden at the coaptation site and to minimize collateral sprouting (35).
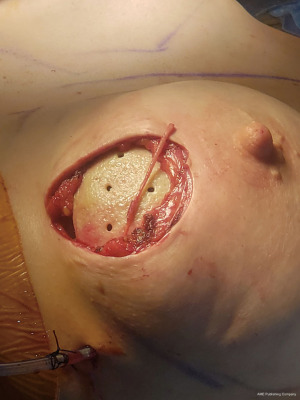
Outcomes measures for sensation have included both neurosensory testing with a pressure-specified sensory device (PSSD) (Axogen, Jacksonville, FL, USA) and patient-reported outcomes regarding nipple and skin flap sensation. Review of outcomes in patients with a minimum of 6 months follow-up shows return of sensation to good or excellent levels in over 80% of patients, which continues to improve at the 1 year follow-up timepoint (unpublished data). Similar results are seen with patient-reported outcomes assessing nipple and overall breast sensation. Importantly, no patients have reported long-term dysethesia, allodynia or symptoms of neuroma formation.
As we have developed our techniques over time, we have worked on optimizing the degree of sensation preservation and timing of return of sensation, as well as better defining patient selection and setting patient expectations. Technical adaptations have included attempting to preserve as much length as possible for nerves that have to be transected in order to allow for shorter nerve allograft reconstruction. While we are still collecting more and longer-term data that will make subgroup analysis possible, early review of our data shows a quicker time to sensory recovery with shorter nerve grafts, consistent with data from upper extremity reconstruction (36,37). As the length of nerve that can be preserved is unpredictable and unable to be determined prior to surgery, patients need to be counseled pre-operatively that implant size may be limited to allow for a tension-free nerve reconstruction with the longest commercially available nerve allograft (7 cm in length). We have found that nerve reconstruction can be reliably performed with implants under 400–450 cc in size, though larger implants may be used for cases of complete nerve preservation or those with nerve reconstruction if longer native nerve lengths can be achieved.
We have found that the technique of nerve preservation during mastectomy and nipple-areolar complex neurotization in the setting of implant-based reconstruction certainly has a learning curve when first implementing the approach into practice. Optimal candidates when first starting would be patients having prophylactic surgery who have not had prior radiation and are not anticipated to have radiation or chemotherapy adjuvantly; patients with a C cup breast or smaller who do not want to be any larger at the completion of their reconstruction; and patients having direct-to-implant reconstruction so the optimal nerve graft length can be determined at the time of surgery. Close partnership and discussion pre-operatively and intra-operatively between the breast and plastic surgeons are also essential to ensure the steps of the procedure are optimized, particularly during dissection of the breast off of the chest wall so that nerve length can be preserved as much as is oncologically possible.
Conclusions
Sensory preservation or restoration after mastectomy is the next frontier in breast reconstruction. Recent advances in the understanding of nerve anatomy, allograft technology, and nerve repair techniques allow surgeons to more widely offer sensation-preserving approaches, though much of the published literature to date has been in the setting of autologous reconstruction. While the data on sensory reinnervation following implant-based reconstruction is still limited, results show promising objective and patient-reported outcomes. Given that the significant majority of breast reconstructions performed are implant-based, expanding these techniques beyond autologous reconstruction is essential to provide more patients the quality-of-life benefits of retaining sensation after mastectomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dung Nguyen) for the series “Cutting-edge of Complex Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-9/coif). The series “Cutting-edge of Complex Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. AWP is a consultant and speaker for Allergan. ZMP is a consultant and speaker for Axogen.The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gass JS, Onstad M, Pesek S, et al. Breast-Specific Sensuality and Sexual Function in Cancer Survivorship: Does Surgical Modality Matter? Ann Surg Oncol 2017;24:3133-40. [Crossref] [PubMed]
- Rabin R. After mastectomies, an unexpected blow: numb new breasts. New York Times. 2017.
- Champaneria MC, Wong WW, Hill ME, et al. The evolution of breast reconstruction: a historical perspective. World J Surg 2012;36:730-42. [Crossref] [PubMed]
- Homsy A, Rüegg E, Montandon D, et al. Breast Reconstruction: A Century of Controversies and Progress. Ann Plast Surg 2018;80:457-63. [Crossref] [PubMed]
- Metcalfe KA, Cil TD, Semple JL, et al. Long-Term Psychosocial Functioning in Women with Bilateral Prophylactic Mastectomy: Does Preservation of the Nipple-Areolar Complex Make a Difference? Ann Surg Oncol 2015;22:3324-30. [Crossref] [PubMed]
- Dossett LA, Lowe J, Sun W, et al. Prospective evaluation of skin and nipple-areola sensation and patient satisfaction after nipple-sparing mastectomy. J Surg Oncol 2016;114:11-6. [Crossref] [PubMed]
- Rodriguez-Unda NA, Bello RJ, Clarke-Pearson EM, et al. Nipple-Sparing Mastectomy Improves Long-Term Nipple But Not Skin Sensation After Breast Reconstruction: Quantification of Long-Term Sensation in Nipple Sparing Versus Non-nipple Sparing Mastectomy. Ann Plast Surg 2017;78:697-703. [Crossref] [PubMed]
- Gahm J, Hansson P, Brandberg Y, et al. Breast sensibility after bilateral risk-reducing mastectomy and immediate breast reconstruction: a prospective study. J Plast Reconstr Aesthet Surg 2013;66:1521-7. [Crossref] [PubMed]
- Yueh JH, Houlihan MJ, Slavin SA, et al. Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg 2009;62:586-90. [Crossref] [PubMed]
- Djohan R, Gage E, Gatherwright J, et al. Patient satisfaction following nipple-sparing mastectomy and immediate breast reconstruction: an 8-year outcome study. Plast Reconstr Surg 2010;125:818-29. [Crossref] [PubMed]
- Peled AW, Duralde E, Foster RD, et al. Patient-reported outcomes and satisfaction after total skin-sparing mastectomy and immediate expander-implant reconstruction. Ann Plast Surg 2014;72:S48-52. [Crossref] [PubMed]
- Jaspars JJ, Posma AN, van Immerseel AA, et al. The cutaneous innervation of the female breast and nipple-areola complex: implications for surgery. Br J Plast Surg 1997;50:249-59. [Crossref] [PubMed]
- Farina MA, Newby BG, Alani HM. Innervation of the nipple-areola complex. Plast Reconstr Surg 1980;66:497-501. [Crossref] [PubMed]
- Riccio CA, Zeiderman MR, Chowdhry S, et al. Plastic Surgery of the Breast: Keeping the Nipple Sensitive. Eplasty 2015;15:e28. [PubMed]
- Schulz S, Zeiderman MR, Gunn JS, et al. Safe Plastic Surgery of the Breast II: Saving Nipple Sensation. Eplasty 2017;17:e33. [PubMed]
- Knackstedt R, Gatherwright J, Cakmakoglu C, et al. Predictable Location of Breast Sensory Nerves for Breast Reinnervation. Plast Reconstr Surg 2019;143:393-6. [Crossref] [PubMed]
- Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg 2014;101:899-911. [Crossref] [PubMed]
- Salibian AA, Frey JD, Choi M, et al. Optimizing the Mastectomy Flap to Improve Aesthetic Outcomes. Aesthet Surg J 2020;40:S1-S12. [Crossref] [PubMed]
- Ducic I, Yoon J, Momeni A, et al. Anatomical Considerations to Optimize Sensory Recovery in Breast Neurotization with Allograft. Plast Reconstr Surg Glob Open 2018;6:e1985. [Crossref] [PubMed]
- Zhou A, Ducic I, Momeni A. Sensory restoration of breast reconstruction - The search for the ideal approach continues. J Surg Oncol 2018;118:780-92. [Crossref] [PubMed]
- Slezak S, McGibbon B, Dellon AL. The sensational transverse rectus abdominis musculocutaneous (TRAM) flap: return of sensibility after TRAM breast reconstruction. Ann Plast Surg 1992;28:210-7. [Crossref] [PubMed]
- Blondeel PN. The sensate free superior gluteal artery perforator (S-GAP) flap: a valuable alternative in autologous breast reconstruction. Br J Plast Surg 1999;52:185-93. [Crossref] [PubMed]
- Blondeel PN, Demuynck M, Mete D, et al. Sensory nerve repair in perforator flaps for autologous breast reconstruction: sensational or senseless? Br J Plast Surg 1999;52:37-44. [Crossref] [PubMed]
- Beugels J, Cornelissen AJM, van Kuijk SMJ, et al. Sensory Recovery of the Breast following Innervated and Noninnervated DIEP Flap Breast Reconstruction. Plast Reconstr Surg 2019;144:178e-88e. [Crossref] [PubMed]
- Spiegel AJ, Menn ZK, Eldor L, et al. Breast Reinnervation: DIEP Neurotization Using the Third Anterior Intercostal Nerve. Plast Reconstr Surg Glob Open 2013;1:e72. [Crossref] [PubMed]
- Weissler JM, Koltz PF, Carney MJ, et al. Sifting through the Evidence: A Comprehensive Review and Analysis of Neurotization in Breast Reconstruction. Plast Reconstr Surg 2018;141:550-65. [Crossref] [PubMed]
- Perdikis G, Chetta M, Kanchwala S, et al. Sensory Recovery after 1 Year from a Multi-Center Prospective Outcomes Registry. American Society for Peripheral Nerve Annual Meeting. Fort Lauderdale, FL. January 2020.
- Peled AW, Peled ZM. Nerve Preservation and Allografting for Sensory Innervation Following Immediate Implant Breast Reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2332. [Crossref] [PubMed]
- Djohan R, Scomacao I, Knackstedt R, et al. Neurotization of the Nipple-Areola Complex during Implant-Based Reconstruction: Evaluation of Early Sensation Recovery. Plast Reconstr Surg 2020;146:250-4. [Crossref] [PubMed]
- Khan A, Zhang J, Sollazzo V, et al. Sensory change of the reconstructed breast envelope after skin-sparing mastectomy. Eur J Surg Oncol 2016;42:973-9. [Crossref] [PubMed]
- Mofid MM, Dellon AL, Elias JJ, et al. Quantitation of breast sensibility following reduction mammaplasty: a comparison of inferior and medial pedicle techniques. Plast Reconstr Surg 2002;109:2283-8. [Crossref] [PubMed]
- Santanelli F, Paolini G, Bittarelli D, et al. Computer-assisted evaluation of nipple-areola complex sensibility in macromastia and following superolateral pedicle reduction mammaplasty: a statistical analysis. Plast Reconstr Surg 2007;119:1679-83. [Crossref] [PubMed]
- Dellon ES, Mourey R, Dellon AL. Human pressure perception values for constant and moving one- and two-point discrimination. Plast Reconstr Surg 1992;90:112-7. [Crossref] [PubMed]
- Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 2009;124:345-53. [Crossref] [PubMed]
- Isaacs J, Safa B, Evans PJ, et al. Technical Assessment of Connector-Assisted Nerve Repair. J Hand Surg Am 2016;41:760-6. [Crossref] [PubMed]
- Isaacs J, Safa B. A Preliminary Assessment of the Utility of Large-Caliber Processed Nerve Allografts for the Repair of Upper Extremity Nerve Injuries. Hand (N Y) 2017;12:55-9. [Crossref] [PubMed]
- Safa B, Jain S, Desai MJ, et al. Peripheral nerve repair throughout the body with processed nerve allografts: Results from a large multicenter study. Microsurgery 2020;40:527-37. [Crossref] [PubMed]
Cite this article as: Peled AW, Peled ZM. Sensory reinnervation after mastectomy with implant-based reconstruction. Ann Breast Surg 2022;6:27.