
Radiation therapy in breast cancer: a narrative review on current standards and future perspectives
Introduction
Breast cancer treatment should be supported by a multidisciplinary team from time of breast cancer diagnosis until end of follow up (1,2), and the team should recommend therapy based on evidence-based guidelines. This approach may not-only increase patient’s satisfaction from treatment but also facilitate treatment decision and management and possibly lead to a better outcome (2). Careful evaluation by the multidisciplinary team including breast radiologists, plastic and breast surgeons, pathologists, radiation, and medical oncologists should guide the treatment approach to improve outcomes (3). Factors such as tumour related findings (e.g., tumour size, molecular subtype), distance of the tumour foci from skin/subcutaneous and/or nipple areola complex, benefit from systemic therapy (pre vs. postoperative), breast size and shape, tumour-size/breast-size ratio and location of the tumour lesion within the breast, patient’s comorbidities, body habitus and contralateral breast shape, patient’s wishes and expectations, and surgeon’s expertise, have significant implications on the treatment approach (3).
Surgical techniques change constantly to improve aesthetic results (4-6). Mastectomy and reconstructive procedures have been refined over the decades, allowing for aesthetic outcomes close to the native breast shape and in symmetry with the contralateral intact breast or even to improve breasts appearance and symmetry (4). Furthermore, in many cases this can be achieved at the time of the mastectomy [i.e., immediate breast reconstruction (IBR)] (7). Nevertheless, the most important notion guiding the team is to maintain oncological safety as a priority and clearly communicate it to the patient. Thus, the treatment approach should not lead to a delay or compromise on oncological treatment (8).
For years, IBR was considered a contraindication if postmastectomy radiation therapy (PMRT) was planned, mainly due to a concern of reconstruction failure and major complications (9,10). Lately, the number of patients receiving PMRT in the setting of IBR increases (11-13). In this changing reality, along with advances in radiation therapy techniques, we should work together to improve PMRT outcomes in the setting of mastectomy and IBR (14,15). The current paper summarizes key principles in radiation therapy and PMRT, considering new surgical techniques for IBR and new, partly experimental PMRT techniques. We present the following article in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-16/rc).
Key principles of current radiation techniques
The key principles for any radiation therapy planning is to clearly define radiation “target volumes” (i.e., areas at risk of subclinical tumour spread), organs at risk (OAR) (i.e., healthy tissues placed in proximity to the target volume whose irradiation could cause damage), dose and fractionation. These should be also applied in the setting of PMRT (16-18).
The radiation oncologist should clearly define the radiation planning objectives, considering patient, disease, and treatment related factors. Patient related factors such as age and comorbidities can dictate the dose constraints to various OARs and/or planning objectives for the target volume coverage (e.g., compromising medial coverage if the tumour bed is lateral, to reduce the cardiac dose) (16,17,19). By performing a mindful physical examination at initial patient visit prior to radiation planning and considering the physical properties of the radiation beam (photons vs. electrons vs. protons), the radiation oncologist can to some extent predict potential side-effects and difficulties in covering target volumes/avoiding OARs (e.g., the area of infra-mammary fold, medial contralateral breast, heart, lung) and which radiation technique should provide a potential advantage in treatment (fewer side effects with adequate target coverage).
Correct delineation of the target volumes in some cases can reduce the OARs doses (20).
When deciding on radiation technique, the radiation oncologist should keep in mind the different dose distribution, low vs. high dose regions and exposure of OARs, and uncertainties in treatment planning, as these may differ significantly by different techniques such as tangential alignment versus volumetric intensity modulated radiation therapy (IMRT) with potential low dose bath. The radiation technique should be decided after considering pro and cons of each approach. A recent publication led by physicists and clinical oncologists from the Danish Breast Cancer Group (DBCG) in collaboration with a multidisciplinary group of international experts nicely shows how different radiation techniques used for planning PMRT cases with implant-based IBR can significantly differ in dose distribution, mainly exposure of OARs, even when planning the same patient case and the same target volumes (16). Therefore, radiation planning should be done meticulously, and decisions should be taken with consideration of disease control and reducing potential toxicity.
PMRT indications and therapeutic value
In the setting of mastectomy, nodal disease is the main indication for PMRT (21). The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis is a landmark publication to establish the role of PMRT in reducing the rate of locoregional recurrences (LRR) as first event after 10-year. The impact of PMRT in reducing the 10-year rate of LRR was correlated with nodal disease stage. For nodal disease stage pN0, the LRR rate was 1.6% for the no-PMRT group versus 3% in the PMRT-group; for the pN1-3 group the LRR rate was 20.3% for the no-PMRT group versus 3.8% for the PMRT group; and for the pN4+ group the LRR rate was 32.1% for the no-PMRT group versus 13% for the PMRT group (21,22). Therefore, for many years, advanced nodal involvement remained the key indication for PMRT (22,23). However, current trials support de-escalation of surgical intervention in patients with low nodal tumour load, and there is an increased application of PMRT to eradicate potential subclinical disease within the regional lymphatics in patients treated with less radical axillary lymph node dissection (24,25). Additionally, there is an increase in the rate of patients who are eligible for breast conserving therapy, but opt for mastectomy and IBR, leading to increased number of PMRT in the setting of IBR. Even though there is no robust data from randomised controlled trials for the use of sentinel node biopsy instead of axillary dissection in mastectomy patients, nor that regional nodal irradiation is sufficient in mastectomy patients with low nodal tumour burden, some of the data guiding this approach is extrapolated from enrolling patients after breast conserving therapy. The landmark EBCTCG PMRT publication (21) also showed the impact of PMRT to the chest wall and regional lymph nodes in 870 patients, with T3 (>5 cm) pN0 who underwent axillary sampling. PMRT to chest wall and regional lymphatics showed statistically significant advantage for reducing the 10-year risk of LRR or any recurrence and a trend towards reducing the breast cancer mortality or any mortality at 20-years. Therefore, along with trials that established the role of regional irradiation instead of axillary dissection in patients with low-nodal disease burden, the EBCTCG subgroup analysis provides additional support for this approach (21).
Furthermore, other clinical and histological factors were suggested to be associated with a high risk for LRR after mastectomy. These include young age at diagnosis (26-29), T3 tumour (30-35), tumour muscle invasion (35,36), high tumour grade (29,35,37), lymphovascular invasion (28,35,37), negative hormone receptor (29-31,38,39), extracapsular nodal tumour extension (32), and a high 21-gene-recurrence score (40,41). Therefore, these factors should be taken into account when considering postoperative radiation but their significance as a sole indicator to support PMRT is not reported in the literature, and therefore unknown.
A thought provoking issue is that in the trials establishing the role of PMRT, the surgical approach included more radical types of mastectomies (i.e., without skin preservation) and axillary clearance (21) thus, less probability for residual breast tissue and less dermal lymphatics (42). Current mastectomy techniques aim to facilitate breast reconstruction by skin sparing (with/without nipple sparing), there is tendency to leave various amounts of residual glandular tissue to facilitate breast reconstruction and allow for better aesthetic outcome of the neo-breast (42). However, as the native skin and subcutaneous tissue are preserved in these surgeries, the dermal plexus, an important lymphatic route for draining the mammary region and may harbour tumour cells, is left intact (43). Thus, the local recurrence risk might be increased in high-risk node-negative patients in which PMRT is not performed (44). Many of the guidelines for breast reconstruction do not provide information in-which cases these procedures should be avoided or in-which PMRT is indicated in patients who are without nodal involvement (45). Using new RT techniques (e.g., imaging-based, deep-inspiration breath hold) and defining the volumes according to ESTRO delineation guidelines (16-18) can contribute reducing the dose to OARs without compromising the target coverage (20). Therefore, the potential therapeutic benefit of PMRT in this setting might be greater comparted to RT based on bony landmarks (46-48). However, PMRT techniques may vary significantly in OAR exposure and target coverage (47,49), and more sophisticated advanced techniques might not necessarily provide an advantage, so careful evaluation of RT plans is recommended. Therefore, it is encouraged to use techniques to reduce the OARs dose such as deep inspiration breath-hold or continuous air way pressure mask (CPAP) and mindfully consider the pros and cons of each RT technique used (47,48). Especially as most of these patients will have a long-term survival which puts them at risk for recurrences or/and RT-related complications.
The target volumes
The target volumes are areas that potentially harbour subclinical disease. Contouring target volumes for chest wall and elective nodal irradiation according to guidelines will help avoiding excessive radiation to adjacent tissues (17,18). In case of IBR, the implant (tissue expander or permanent implant) may be positioned ventral or dorsal to the major pectoral muscle. The ESTRO-ACROP guidelines for PMRT in early breast cancer indicate that the target volume includes the residual subcutaneous glandular tissue and the subcutaneous lymphatics and that the major pectoral muscle serves as the anatomical dorsal border for mastectomy. Therefore, the breast glandular tissue position is dependent of the implant location. In case of muscle invasion, local inclusion of that part of the pectoral muscle is advised, and in case of rib cage invasion the ribs/intercostal muscles should also be focally included in the target volume, although these patients are usually not candidates for IBR (17). We recommend using these guidelines when planning early breast cancer radiation therapy, but the delineation should be adopted per case accordingly, using available preoperative/pre-systemic therapy imaging for planning and identifying the risk areas for recurrence.
The timing of PMRT in the setting of reconstruction
Reconstructions can be immediate, delayed, or delayed–immediate. Immediate reconstructions are performed at mastectomy, whereas delayed reconstructions are usually performed 6–12 months (or years) after the completion of mastectomy and adjuvant therapy, when the patient is recovered from treatment related toxicity (50). Different factors dictate the timing of reconstruction (50). Immediate reconstruction is facilitated by skin sparing (SSM) or nipple sparing mastectomy (NSM, i.e., sparing of the skin and nipple and areola complex). By contrast, delayed-breast reconstruction was the common approach after non-skin sparing procedure, especially if patients were planned for PMRT prior to surgery. This approach allowed for the irradiated skin to be replaced with healthy skin from a donor site.
Delayed-immediate reconstruction involves placing tissue expanders at the time of mastectomy (50). This may allow to maintain or expand the skin and pectoralis muscle to create a pocket for the implant. Additionally, the decision on PMRT can be based on the final pathology report. Usually, patients not planned for PMRT complete reconstruction with an implant or flap, whereas patients planned for PMRT undergo PMRT with a tissue expander followed by later definitive reconstruction. The immediate-delayed approach permits the opportunity to avoid irradiating an autologous flap (if planned), gradually expand the pectoralis muscle to serve as a pocket for a permanent implant, and the benefits of providing an immediate breast mound for the patient after mastectomy.
In the past, immediate reconstruction was considered contraindicated if PMRT was planned, however, recently more studies are reporting its use (11,12,50).
Unfortunately, there is no consensus regarding the timing of the reconstruction (immediate, delayed, or delayed-immediate) in the setting of PMRT and the treatment approach varies significantly among centres and countries. The rate of reconstruction failure varies substantially from 0% to 40%, depending on whether PMRT was delivered to the tissue expander or to the permanent implant. Recent publications suggest that PMRT to tissue expander is associated with a higher rate of complications while others did not find significant differences (51-53).
Therefore, further trials are needed to determine the optimal approach for reconstruction in the setting of PMRT with regards to timing if a two-stage expander/implant reconstruction is planned.
Bolus
Bolus was commonly used for PMRT chest wall irradiation (without reconstruction) to serve as a tissue equivalent material placed on the skin to shift the 95–100% isodose line to the skin and subcutis to reduce the local recurrences in these volumes (54). However, bolus was the most important independent risk factor for severe skin toxicity in case of PMRT without strong evidence for lower rates of local recurrence (55,56). Importantly, its use in the setting of SSM/NSM, varies between institutions, and little data is available with regards to complications/failure of the reconstruction (55,56). Therefore, until further data become available, the routinely use of a bolus in these cases is not recommended and should be considered on an individual basis if there is a concern for a high-risk area that is not getting full coverage (55,56).
Radiation boost
Historically, radiation boost in the setting of PMRT was aimed to provide an additional radiation dose to the mastectomy scar to reduce local recurrences in this area (57). A study by Massachusetts General Hospital (57), evaluated whether a chest wall boost was independently associated with reconstruction complications in the setting of breast reconstruction. The study cohort included patients who had delayed reconstruction procedures. Radiation boost was significantly associated with infection, skin necrosis, and implant exposure. For implant-based reconstruction, the addition of the boost was independently associated with higher risks of implant failure. Most importantly, the addition of the boost was not associated with improving local tumour control, even in high-risk subgroups (57). Therefore, we do not recommend routine use of boost in case of IBR.
Dose and fractionation
Practice patterns vary widely among centres and countries with regards to total dose and fractionation schedule for breast cancer patients who underwent mastectomy with/without IBR. The most common used fraction sizes in case of IBR is 1.8–2 Gy to a total dose of 50–50.4 Gy (58). However, some countries adopted the moderate hypofractionation regimens (e.g., 40 Gy delivered in 15 fractions over 3 weeks) to the chest wall and regional nodes, even in the setting of IBR, based on long-term data from the START A/B trials, showing reduced toxicity of hypofractionation scheme compared to normo-fractionation (1.8–2 Gy per fraction to 50–50.4 Gy) (59). Even though there is little data from clinical trials specifically evaluating hypofractionation in the setting of IBR to support its use, and there are several ongoing clinical trials, based on the long-term data of hypofractionation in breast conserving therapy, there is no reason to believe that its outcome will be inferior to conventional fractionation (58-61).
Proton-based RT
Proton therapy has not been widely used nor investigated for adjuvant breast cancer RT, because there are only few proton centers across the world. However, due to the properties of proton therapy it is possible to achieve optimal dose coverage of relevant targets and at the same time ensure low dose to OAR compared with photon RT. The use of volumetric based-photon planning (i.e., arc-based intensity modulated radiation therapy, vIMRT) for breast cancer might not achieve dosimetric advantage over tangential field-based planning (49). The use of vIMRT often results in large volumes receiving a low dose “bath”, which may result in unexpected toxicity (if these organs were not contoured and taken into consideration while planning) (62), and possibility for secondary cancer as many of these patients are long-term survivors (63).
In an energy-dependent manner, proton therapy will deposit the majority of its dose in tissue depths defined by the Bragg peak (64). In practice, this translates into (I) the ability to deliver the peak energy to target volumes of irregular 3-dimensional shape using pencil-beam scanning technology, (II) a sharp dose fall-off following deposition of energy in the target and (III) reduction of the integral dose to the patient. Within millimeters, the exit dose drops off from 90% to 10%, resulting in the virtual absence of an exit dose. The effectiveness, safety and feasibility of proton therapy has been reported in few small cohort studies with limited follow up, and there is a lack of clinically controlled randomised trials documenting benefit from proton therapy, evaluated either as higher tumour control and/or as fewer morbidities.
The potential of proton therapy for PMRT is to lower the dose to heart and lung without a compromise on dose to chest wall target on regional nodes. However, proton therapy has an estimated 10% higher radiobiologic effective dose (RBE), and studies imply that the relative effect may be even higher, leading to a higher risk of morbidities from OAR than anticipated (65). Most studies on proton therapy in early breast cancer have been single-institution and retrospective with no formal research plan (66,67), but fortunately, well-designed trials are also made. Seventy patients requiring loco-regional RT including internal mammary node irradiation were treated with proton therapy in a phase II trial from Boston 2011–2016 (68). Inclusion criteria were >20 Gy was received by >5% of the heart or >20 Gy to the left anterior descending artery with conventional photon RT. The doses were 1.8–2.0 Gy (RBE), 25–28 fractions. The primary endpoint was grade 3 or worse radiation pneumonitis or any grade 4 toxicity within 3 months from proton therapy. Mastectomy was done in 93%, and 83% of these pursued reconstructions. At median 55 months follow-up, and the 5-yr LRR and OS were 1.5% and 91%, respectively, and only one patient developed grade 2 pneumonitis as the highest morbidity score. As of 2021, there are 2 phase III randomised controlled clinical trials investigating gain and risk from proton therapy in breast cancer patients. The RadComp trial (NCT 02603341) is a pragmatic randomised trial testing proton vs. photon RT for patients with stage II-III breast cancer with an indication for loco-regional RT including internal mammary node irradiation (69). The primary endpoint is major coronary event reduction by proton therapy, hypothesizing a reduction in the 10-year major coronary events rate from 6.3% to 3.8% compared to photons. The trial aims for 1,278 patients accrued during 2016–2022. The other trial open for inclusion since 2020 is the DBCG Proton trial (NCT04291378), where patients operated for breast cancer or DCIS can be included if photon treatment planning with strict criteria for dose coverage of breast, chest wall and nodal volumes reveals a mean heart dose ≥4 Gy and/or V20lung ≥37% (trial protocol is available on Google). The primary endpoint is 10-year risk of heart disease, hypothesizing a 10-year reduction from 10.2% (photon) to 6.3% (proton). The baseline 10-year risk of heart disease in Danish women 60 years old is 5.8%. The trial aims for 1,502 patients. Both the RadComp and the DBCG trials have several secondary endpoints including extensive reporting of loco-regional radiation associated morbidities and documenting the pattern of recurrence.
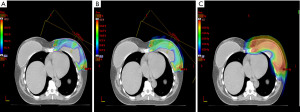
Since proton therapy requires a higher precision in daily therapy due to the properties of the beam (Figure 1 to show en face beam arrangement and dose very close to heart), and one of the main reasons for using proton therapy in breast cancer is concern of heart disease, it is likely that future reporting of results from proton trials will include reporting of doses to substructures of the heart. An automated atlas for delineating 25 substructures of the heart has been reported from Denmark, but other countries are likely to develop similar atlases (70). However, providing RT on a single planning-CT-scan according to strict institutional guidelines does not guarantee that the treatment is reproducible. For example, by using cine images recorded during each radiation fraction, it is possible to detect a quite substantial variation in the heart position in some patients, whilst for other patients the position of the heart is robust during the whole treatment period (71).
Future trials
Currently there are several trials aiming to improve the outcomes of patients who are planned for mastectomy, reconstruction and are candidates for PMRT (Table 1). Some are aimed to evaluate the fractionation protocols as FABREC (NCT03422003) and RTCharm (NCT03414970) that are planned to compare conventional vs. hypofractionated regimens in breast cancer patients with IBR. The DBCG RT Recon trial is aimed to evaluate the timing of reconstruction (immediate vs. immediate-delayed) and fractionation (allows for conventional and moderate-hypofractionation). While trials such as Primary Radiotherapy And DIEP flAp Reconstruction Trial (PRADA) (NCT02771938), aim to evaluate preoperative radiation in patients who are planned for mastectomy and autologous-based reconstruction.
Table 1
| Trial name (NCT) | Accrual targets (number of patients) | Accrual study start year | Design | Primary end point |
|---|---|---|---|---|
| DBCG RT Recon trial NCT03730922 | 590 | 2020 | Prospective randomized | Surgical complications of immediate-delayed versus delayed reconstruction in patients who are planned for PMRT |
| FABREC (NCT03422003) | 400 | 2018 | Prospective randomized | Patient reported outcomes (note: reconstruction complications and oncological outcome are secondary endpoints) |
| RTCharm (NCT03414970) | 880 | 2018 | Prospective randomized | To evaluate whether the reconstruction complication rate at 24 months post radiation is non-inferior with hypofractionation |
| Primary Radiotherapy And DIEP flAp Reconstruction Trial (PRADA) (NCT02771938) | 60 | 2016 | Interventional non-randomized | Number of participants with presence of open breast wound at 4 weeks after DIEP surgery |
NCT, ClinicalTrials.gov Identifier; PMRT, postmastectomy radiation therapy; DIEP, deep inferior epigastric perforator.
Conclusions
Breast cancer treatment evolved significantly with improvement in surgical and RT techniques. Radiation planning should be guided by disease stage, risk of recurrence, correct definition of the target volumes and treatment objectives. Meticulous RT planning, total dose and fractionation, dose homogeneity, and OAR doses are significant for reducing RT toxicity. The multidisciplinary team should work together in aim to improve the outcomes of mastectomy patients in both in clinic and in planning future trials.
Acknowledgments
Funding: Birgitte Vrou Offersen was funded by the Danish Cancer Society and the Danish Comprehensive Cancer Center Radiotherapy Group and the Novo Nordisk Foundation.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction - The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-16/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-16/coif). The series “Breast Reconstruction - The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gabel M, Hilton NE, Nathanson SD. Multidisciplinary breast cancer clinics. Do they work? Cancer 1997;79:2380-4. [Crossref] [PubMed]
- Dubsky P, Pinker K, Cardoso F, et al. Breast conservation and axillary management after primary systemic therapy in patients with early-stage breast cancer: the Lucerne toolbox. Lancet Oncol 2021;22:e18-28. [Crossref] [PubMed]
- Akhtar Z, Stearns V, Cartwright P, et al. The effect of 1-day multidisciplinary clinic on breast cancer treatment. Breast Cancer Res Treat 2020;182:623-9. [Crossref] [PubMed]
- Macadam SA, Bovill ES, Buchel EW, et al. Evidence-Based Medicine: Autologous Breast Reconstruction. Plast Reconstr Surg 2017;139:204e-29e. [Crossref] [PubMed]
- Ho AL, Tyldesley S, Macadam SA, et al. Skin-sparing mastectomy and immediate autologous breast reconstruction in locally advanced breast cancer patients: a UBC perspective. Ann Surg Oncol 2012;19:892-900. [Crossref] [PubMed]
- Wilkins EG, Hamill JB, Kim HM, et al. Complications in Postmastectomy Breast Reconstruction: One-year Outcomes of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study. Ann Surg 2018;267:164-70. [Crossref] [PubMed]
- Scheflan M, Allweis TM, Ben Yehuda D, et al. Meshed Acellular Dermal Matrix in Immediate Prepectoral Implant-based Breast Reconstruction. Plast Reconstr Surg Glob Open 2020;8:e3265. [Crossref] [PubMed]
- Lee KT, Jung JH, Mun GH, et al. Influence of complications following total mastectomy and immediate reconstruction on breast cancer recurrence. Br J Surg 2020;107:1154-62. [Crossref] [PubMed]
- Kelley BP, Ahmed R, Kidwell KM, et al. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: are current practices ideal? Ann Surg Oncol 2014;21:1732-8. [Crossref] [PubMed]
- Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol 2014;21:118-24. [Crossref] [PubMed]
- Cooke AL, Diaz-Abele J, Hayakawa T, et al. Radiation Therapy Versus No Radiation Therapy to the Neo-breast Following Skin-Sparing Mastectomy and Immediate Autologous Free Flap Reconstruction for Breast Cancer: Patient-Reported and Surgical Outcomes at 1 Year-A Mastectomy Reconstruction Outcomes Consortium (MROC) Substudy. Int J Radiat Oncol Biol Phys 2017;99:165-72. [Crossref] [PubMed]
- Heiman AJ, Gabbireddy SR, Kotamarti VS, et al. A Meta-Analysis of Autologous Microsurgical Breast Reconstruction and Timing of Adjuvant Radiation Therapy. J Reconstr Microsurg 2021;37:336-45. [Crossref] [PubMed]
- Naoum GE, Salama L, Niemierko A, et al. Single Stage Direct-to-Implant Breast Reconstruction Has Lower Complication Rates Than Tissue Expander and Implant and Comparable Rates to Autologous Reconstruction in Patients Receiving Postmastectomy Radiation. Int J Radiat Oncol Biol Phys 2020;106:514-24. [Crossref] [PubMed]
- Kaidar-Person O, Hermann N, Poortmans P, et al. A multidisciplinary approach for autologous breast reconstruction: A narrative (re)view for better management. Radiother Oncol 2021;157:263-71. [Crossref] [PubMed]
- Kaidar-Person O, Offersen BV, Boersma LJ, et al. A multidisciplinary view of mastectomy and breast reconstruction: Understanding the challenges. Breast 2021;56:42-52. [Crossref] [PubMed]
- Kaidar-Person O, Vrou Offersen B, Hol S, et al. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol 2019;137:159-66. [Crossref] [PubMed]
- Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother Oncol 2016;118:205-8. [Crossref] [PubMed]
- Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 2015;114:3-10. [Crossref] [PubMed]
- Raj KA, Evans ES, Prosnitz RG, et al. Is there an increased risk of local recurrence under the heart block in patients with left-sided breast cancer? Cancer J 2006;12:309-17. [Crossref] [PubMed]
- Chang KH, Chang JS, Park K, et al. A Retrospective Dosimetric Analysis of the New ESTRO-ACROP Target Volume Delineation Guidelines for Postmastectomy Volumetric Modulated Arc Therapy After Implant-Based Immediate Breast Reconstruction. Front Oncol 2020;10:578921. [Crossref] [PubMed]
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [Crossref] [PubMed]
- Kaššák F, Rossier C, Picardi C, et al. Postmastectomy radiotherapy in T1-2 patients with one to three positive lymph nodes - Past, present and future. Breast 2019;48:73-81. [Crossref] [PubMed]
- Recht A, Comen EA, Fine RE, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Ann Surg Oncol 2017;24:38-51. [Crossref] [PubMed]
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Sávolt Á, Péley G, Polgár C, et al. Eight-year follow up result of the OTOASOR trial: The Optimal Treatment Of the Axilla - Surgery Or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: A randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol 2017;43:672-9. [Crossref] [PubMed]
- Matthews RH, McNeese MD, Montague ED, et al. Prognostic implications of age in breast cancer patients treated with tumorectomy and irradiation or with mastectomy. Int J Radiat Oncol Biol Phys 1988;14:659-63. [Crossref] [PubMed]
- Laurberg T, Alsner J, Tramm T, et al. Impact of age, intrinsic subtype and local treatment on long-term local-regional recurrence and breast cancer mortality among low-risk breast cancer patients. Acta Oncol 2017;56:59-67. [Crossref] [PubMed]
- Buchanan CL, Dorn PL, Fey J, et al. Locoregional recurrence after mastectomy: incidence and outcomes. J Am Coll Surg 2006;203:469-74. [Crossref] [PubMed]
- Geurts YM, Witteveen A, Bretveld R, et al. Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res Treat 2017;165:709-20. [Crossref] [PubMed]
- Pisansky TM, Ingle JN, Schaid DJ, et al. Patterns of tumor relapse following mastectomy and adjuvant systemic therapy in patients with axillary lymph node-positive breast cancer. Impact of clinical, histopathologic, and flow cytometric factors. Cancer 1993;72:1247-60. [Crossref] [PubMed]
- Recht A, Gray R, Davidson NE, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol 1999;17:1689-700. [Crossref] [PubMed]
- Jager JJ, Volovics L, Schouten LJ, et al. Loco-regional recurrences after mastectomy in breast cancer: prognostic factors and implications for postoperative irradiation. Radiother Oncol 1999;50:267-75. [Crossref] [PubMed]
- Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641-8. [Crossref] [PubMed]
- Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949-55. [Crossref] [PubMed]
- Nielsen HM, Overgaard M, Grau C, et al. Loco-regional recurrence after mastectomy in high-risk breast cancer--risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol 2006;79:147-55. [Crossref] [PubMed]
- Fowble B, Gray R, Gilchrist K, et al. Identification of a subgroup of patients with breast cancer and histologically positive axillary nodes receiving adjuvant chemotherapy who may benefit from postoperative radiotherapy. J Clin Oncol 1988;6:1107-17. [Crossref] [PubMed]
- O'Rourke S, Galea MH, Morgan D, et al. Local recurrence after simple mastectomy. Br J Surg 1994;81:386-9. [Crossref] [PubMed]
- Fisher BJ, Perera FE, Cooke AL, et al. Long-term follow-up of axillary node-positive breast cancer patients receiving adjuvant systemic therapy alone: patterns of recurrence. Int J Radiat Oncol Biol Phys 1997;38:541-50. [Crossref] [PubMed]
- Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol 2011;29:2852-8. [Crossref] [PubMed]
- Woodward WA, Barlow WE, Jagsi R, et al. Association Between 21-Gene Assay Recurrence Score and Locoregional Recurrence Rates in Patients With Node-Positive Breast Cancer. JAMA Oncol 2020;6:505-11. [Crossref] [PubMed]
- Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol 2010;28:1677-83. [Crossref] [PubMed]
- Kaidar-Person O, Boersma LJ, Poortmans P, et al. Residual Glandular Breast Tissue After Mastectomy: A Systematic Review. Ann Surg Oncol 2020;27:2288-96. [Crossref] [PubMed]
- Kaidar-Person O, Poortmans P, Offersen BV, et al. Spatial location of local recurrences after mastectomy: a systematic review. Breast Cancer Res Treat 2020;183:263-73. [Crossref] [PubMed]
- Bernstein-Molho R, Laitman Y, Galper S, et al. Locoregional Treatments and Ipsilateral Breast Cancer Recurrence Rates in BRCA1/2 Mutation Carriers. Int J Radiat Oncol Biol Phys 2021;109:1332-40. [Crossref] [PubMed]
- Kaidar-Person O, Poortmans P, Offersen BV, et al. What are the guidelines for immediate breast reconstruction? Eur J Surg Oncol 2021;47:1214-5. [Crossref] [PubMed]
- Bartlett FR, Colgan RM, Carr K, et al. The UK HeartSpare Study: randomised evaluation of voluntary deep-inspiratory breath-hold in women undergoing breast radiotherapy. Radiother Oncol 2013;108:242-7. [Crossref] [PubMed]
- Reckhow J, Kaidar-Person O, Ben-David MA, et al. Continuous positive airway pressure with deep inspiration breath hold in left-sided breast radiation therapy. Med Dosim 2021;46:127-31. [Crossref] [PubMed]
- Drost L, Yee C, Lam H, et al. A Systematic Review of Heart Dose in Breast Radiotherapy. Clin Breast Cancer 2018;18:e819-24. [Crossref] [PubMed]
- Kaidar-Person O, Nissen HD, Yates ES, et al. Postmastectomy Radiation Therapy Planning After Immediate Implant-based Reconstruction Using the European Society for Radiotherapy and Oncology-Advisory Committee in Radiation Oncology Practice Consensus Guidelines for Target Volume Delineation. Clin Oncol (R Coll Radiol) 2021;33:20-9. [Crossref] [PubMed]
- Ho AY, Hu ZI, Mehrara BJ, et al. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol 2017;18:e742-53. [Crossref] [PubMed]
- Cordeiro PG, Albornoz CR, McCormick B, et al. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg 2014;134:588-95. [Crossref] [PubMed]
- Santosa KB, Chen X, Qi J, et al. Postmastectomy Radiation Therapy and Two-Stage Implant-Based Breast Reconstruction: Is There a Better Time to Irradiate? Plast Reconstr Surg 2016;138:761-9. [Crossref] [PubMed]
- Lee KT, Mun GH. Optimal Sequencing of Postmastectomy Radiotherapy and Two Stages of Prosthetic Reconstruction: A Meta-analysis. Ann Surg Oncol 2017;24:1262-8. [Crossref] [PubMed]
- Shiba S, Okamoto M, Kiyohara H, et al. Clinical Advantage of Chest-wall Post-mastectomy Radiation Therapy Without Bolus. In Vivo 2018;32:961-5. [Crossref] [PubMed]
- Dahn HM, Boersma LJ, de Ruysscher D, et al. The use of bolus in postmastectomy radiation therapy for breast cancer: A systematic review. Crit Rev Oncol Hematol 2021;163:103391. [Crossref] [PubMed]
- Nichol A, Narinesingh D, Raman S, et al. The Effect of Bolus on Local Control for Patients Treated With Mastectomy and Radiation Therapy. Int J Radiat Oncol Biol Phys 2021; Epub ahead of print. [Crossref] [PubMed]
- Naoum GE, Salama L, Ho A, et al. The Impact of Chest Wall Boost on Reconstruction Complications and Local Control in Patients Treated for Breast Cancer. Int J Radiat Oncol Biol Phys 2019;105:155-64. [Crossref] [PubMed]
- Marta GN, Coles C, Kaidar-Person O, et al. The use of moderately hypofractionated post-operative radiation therapy for breast cancer in clinical practice: A critical review. Crit Rev Oncol Hematol 2020;156:103090. [Crossref] [PubMed]
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-94. [Crossref] [PubMed]
- Offersen BV, Alsner J, Nielsen HM, et al. Hypofractionated Versus Standard Fractionated Radiotherapy in Patients With Early Breast Cancer or Ductal Carcinoma In Situ in a Randomized Phase III Trial: The DBCG HYPO Trial. J Clin Oncol 2020;38:3615-25. [Crossref] [PubMed]
- Song SY, Chang JS, Fan KL, et al. Hypofractionated Radiotherapy With Volumetric Modulated Arc Therapy Decreases Postoperative Complications in Prosthetic Breast Reconstructions: A Clinicopathologic Study. Front Oncol 2020;10:577136. [Crossref] [PubMed]
- Kaidar-Person O, Kostich M, Zagar TM, et al. Helical tomotherapy for bilateral breast cancer: Clinical experience. Breast 2016;28:79-83. [Crossref] [PubMed]
- Grantzau T, Overgaard J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: A systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother Oncol 2016;121:402-13. [Crossref] [PubMed]
- Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 2014;59:R419-72. [Crossref] [PubMed]
- Wang CC, McNamara AL, Shin J, et al. End-of-Range Radiobiological Effect on Rib Fractures in Patients Receiving Proton Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys 2020;107:449-54. [Crossref] [PubMed]
- Luo L, Cuaron J, Braunstein L, et al. Early outcomes of breast cancer patients treated with post-mastectomy uniform scanning proton therapy. Radiother Oncol 2019;132:250-6. [Crossref] [PubMed]
- Kammerer E, Guevelou JL, Chaikh A, et al. Proton therapy for locally advanced breast cancer: A systematic review of the literature. Cancer Treat Rev 2018;63:19-27. [Crossref] [PubMed]
- Jimenez RB, Hickey S, DePauw N, et al. Phase II Study of Proton Beam Radiation Therapy for Patients With Breast Cancer Requiring Regional Nodal Irradiation. J Clin Oncol 2019;37:2778-85. [Crossref] [PubMed]
- Bekelman JE, Lu H, Pugh S, et al. Pragmatic randomised clinical trial of proton versus photon therapy for patients with non-metastatic breast cancer: the Radiotherapy Comparative Effectiveness (RadComp) Consortium trial protocol. BMJ Open 2019;9:e025556. [Crossref] [PubMed]
- Milo MLH, Offersen BV, Bechmann T, et al. Delineation of whole heart and substructures in thoracic radiation therapy: National guidelines and contouring atlas by the Danish Multidisciplinary Cancer Groups. Radiother Oncol 2020;150:121-7. [Crossref] [PubMed]
- Poulsen PR, Thomsen MS, Hansen R, et al. Fully automated detection of heart irradiation in cine MV images acquired during breast cancer radiotherapy. Radiother Oncol 2020;152:189-95. [Crossref] [PubMed]
Cite this article as: Kaidar-Person O, Offersen BV. Radiation therapy in breast cancer: a narrative review on current standards and future perspectives. Ann Breast Surg 2022;6:28.

