Breast reinnervation—the next frontier in autologous breast reconstruction: a review of early results
Introduction
Breast cancer has been one of the foremost cancer diagnoses and leading causes of cancer mortality among women for decades, resulting in more than 40,000 deaths annually (1).Nearly 13% of women will be diagnosed with breast cancer during their lifetime (2). Fortunately, continued advances in medical and surgical oncological practices have improved 5-year average survival rates to 90.0%, allowing for greater focus on minimizing the morbidities associated with breast cancer (3).
As rates of prophylactic mastectomies increase across the United States, including contralateral prophylactic mastectomies, increased attention has been given to refining reconstructive techniques to improve both aesthetic and functional outcomes, with surgeons exploring new methods by which to address areas of discontent and improve patient reported outcomes (4-11). The lack of sensation after breast reconstruction is one such arena.
While improvements have been made to the aesthetic form of the reconstructed breast, studies show that a major cause of patient dissatisfaction after breast reconstruction is the unnatural decreased sensation of the reconstructed breast (12-14). Autologous breast reconstruction has been shown to fare better than implant-based reconstruction in this regard, and sensibility of free flap breast reconstruction has been shown to improve patient rated quality of life (4,13,15-21).Studies from the 1980s were able to demonstrate that spontaneous recovery of sensation from both the skin margins and the deep surface of the flap is possible in autologous breast reconstruction (12,19,22-24). However, this recovered sensation is quite limited, and there have been documented injuries including thermal injuries sustained by patients who do not regain protective sensation and the ability to respond to nocuous stimuli to the breast (25-28). Surgical repair of the affected nerves has been shown to increase not only the quality and quantity of recovered sensation, but also the likelihood of recovery of erogenous sensation to the remaining breast tissue (22,25,29-31).
Nerves can be repaired through direct coaptation or with the use of a conduit or graft, which serve as a scaffold to guide nerve regeneration and growth (32).In autologous breast reconstruction, a tension free coaptation of donor nerves within the flap to recipient intercostal nerves within the chest can be limited by the availability of adequate length and quality of the cutaneous nerves at both sites requiring increased surgical dissection of the nerves within the rectus abdominis muscle in order to obtain greater length of the donor nerve within the flap. This extensive dissection can weaken and injure the remaining muscle (22,31). To mitigate abdominal wall morbidity caused by harvesting increasing lengths of flap donor nerve, nerve allografts are used to provide the length necessary for coapting the donor and recipient nerves with little to no tension (31-33). The use of nerve allografts over autografts is supported by reduced donor site morbidity, as allografts are readily available, and show comparable clinical outcomes. The authors describe a surgical technique for using an interpositional nerve allograft as a method of breast innervation in autologous breast reconstruction using abdominal free tissue transfer and share their early results using nerve allograft.
Methods
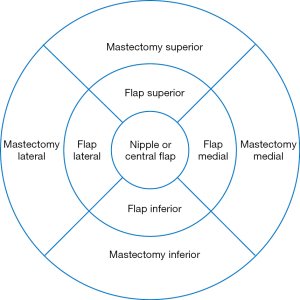
A retrospective chart review of patients undergoing post mastectomy autologous breast reconstruction by the senior author (MDC) was conducted. Females greater than 18 years of age who had undergone post mastectomy autologous breast reconstruction within the previous 18 months and were able to provide informed consent were included in the study. Demographic information, mastectomy and reconstructive surgical details, and post-operative sensory outcomes were recorded. Sixty-seven autologous free flaps were performed on 52 enrolled patients between 2017 and 2019. Forty flaps underwent neurotization using nerve allograft while twenty-seven flaps were not neurotized and thus serve as controls. Neurotizations were performed to the third or fourth intercostal nerve by coaptation to the flap donor nerve with the allograft assisted with a nerve connector consisting of porcine submucosal extracellular matrix. Recovery of sensation to nine previously described areas of the flap and mastectomy skin were measured with Semmes Weinstein monofilaments post operatively at 3, 6, and 12 months in order to determine incidence of recovered sensation, area of recovered sensation, and quality of recovered sensation (Figure 1).
Surgical technique
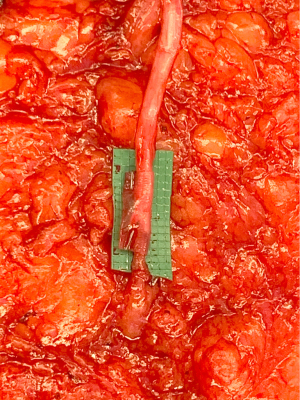
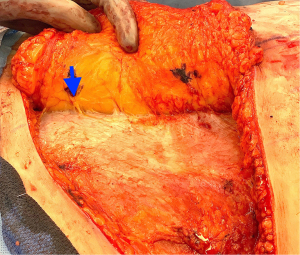
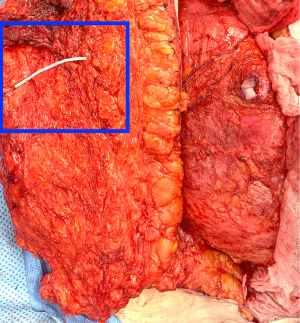
During the standard elevation of the abdominal flap and identification of the major skin perforators from the deep inferior epigastric artery, thoracic intercostal nerves are also identified at the T10, T11, and T12 levels (Figure 2). To ensure that only the sensory component of the nerve is utilized and that the motor branches to the rectus muscle are maintained, the donor nerve is taken at the level of the anterior abdominal fascia. Once the nerve has pierced the fascia the composition is purely sensory, whereas the nerve is mixed and comprised of both sensory and motor components below the level of the fascia (22,31). After the donor intercostal nerve on the abdominal flap is identified, a recipient nerve in the breast cavity must also be identified. The recipient nerves are the anterior cutaneous branches of the third or fourth intercostal nerves or the anterior branches of the lateral cutaneous branches of the third or fourth intercostal nerves encountered along the medial or lateral aspect of the breast cavity after mastectomy, respectively. The anterior cutaneous branch of the intercostal nerve is easily encountered along the medial aspect of the breast cavity during rib harvest as it travels consistently along the inferior border of the rib external to the perichondrium and crosses over the internal mammary artery and vein (Figure 3). The surgeon can further dissect out this nerve laterally along the rib to obtain additional length for coaptation. The anterior branch of the lateral cutaneous branch of the intercostal nerve is often found along the lateral border of the pectoralis major muscle and can be further dissected into the muscle for additional length (Figure 4) (31). When possible, the anterior cutaneous branch of the lateral fourth intercostal nerve is selected given its significant contribution in providing sensation to the nipple areolae complex in the native breast, however selection of the recipient nerve is ultimately based on ease of dissection, identification, and proximity to the donor nerve within the abdominal flap after anticipated flap inset to ensure a tension-free coaptation. Collaboration with breast surgeons to identify and dissect the nerves from the breast specimen at the time of mastectomy may further facilitate and expedite neurotization.

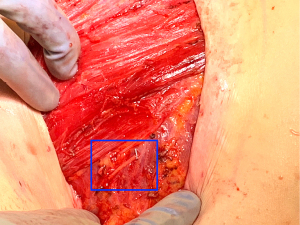
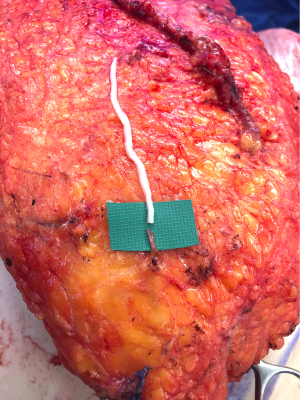
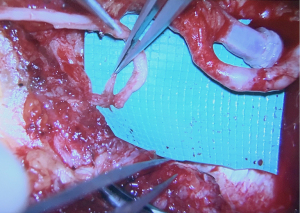
Once the donor and recipient nerves have been prepared, there is often an expected gap which may be overcome by placement of an interpositional nerve allograft with sufficient length to span the anticipated gap. To minimize ischemia time, the nerve allograft is coapted to the donor nerve within the abdominal flap in situ prior to transfer and vascular anastomosis (Figure 5). The nerve and allograft are coapted by with two handsewn epineural interrupted sutures using 9-0 nylon under Loupe magnification (Figure 6). The authors utilize a nerve connector made from porcine submucosa to facilitate the nerve repair, as several studies have shown that connectors can improve directed nerve regeneration across the coaptation site (Figure 7) (30-32,34).Following vascular anastomosis, the recipient intercostal nerve is then coapted to the nerve allograft, which is coapted to the donor nerve within the abdominal flap. If the anterior intercostal nerve branch in the medial breast cavity is selected as the recipient nerve then the coaptation is performed within the same operative field as the vascular anastomosis (Figure 8). If the lateral cutaneous intercostal nerve in the lateral breast cavity is selected as a recipient nerve, then the coaptation may be performed by reflecting the flap medially with the use of a surgical assistant. The coaptation is again assisted with the nerve connector and the flap is then inset, taking care to avoid tension on the repair. As with any new surgical technique there is a learning curve to performing neurotization, but with familiarity, this procedure may be completed within minutes.
Results and discussion
Forty-nine flaps (29 neurotized and 20 non-neurotized flaps) had greater than 6 months follow up. Initial results demonstrated a higher incidence of recovered sensation and a greater area of recovered sensation in neurotized flaps, however this difference narrowed toward the 12 month follow up. After 12 months, 93% of the neurotized flaps showed evidence of recovered sensation compared with only 87% of non-neurotized flaps. The average number or areas with recovered sensation in neurotized and non-neurotized flaps had sensation recovery to an average of 2 of the 9 previously described areas of the flap and mastectomy skin. There was a slight difference in the return of protective sensation and sensation density as evidenced by the Semmes-Weinstein monofilament test of between the neurotized and non-neurotized flaps, however, this difference was not clinically significant (5.18 and 5.43 g respectively).
Several studies have shown that sensation after autologous breast reconstruction is improved after neurotization, with return of sensation occurring at an accelerated rate and with greater quality and in greater quantity (12-13,22,30,35). Blondeel was one of the earliest to demonstrate improved sensory recovery outcomes after pure sensory coaptation. He studied 121 breasts comprised of 56 nonoperated breasts, 24 neurotized DIEP flaps, 13 non-neurotized DIEP flaps, and 28 non-neurotized TRAM flaps and evaluated quality of recovered sensation using Semmes–Weinstein monofilaments to measure detection of pressure and metal probes to ascertain hot and cold recognition, as well as through sensory evoked potentials. Results showed that 75% of neurotized DIEP flaps regained protective sensation and this sensation was present in all five segments of the breast, compared with 31% of non-neurotized DIEP flaps and 18% of non-neurotized TRAM flaps. The neurotized flaps also demonstrated greater return of erogenous sensation compared with the non-neurotized groups and recovered sensation at lower pressure thresholds (22). More recently, a retrospective study by Speigel showed that of 57 DIEP flaps (9 non-neurotized controls, 48 neurotized flaps of which 33 neurotizations were performed with a 4-cm conduit and 15 neurotizations were performed by direct coaptation), neurotization with nerve conduit achieved recovery of sensation at significantly lower pressure thresholds as compared with flaps neurotized by direct coaptation (30).This finding is noteworthy as conduits are often touted as being most beneficial for noncritical gaps of less than 1 cm (32,36).Improved sensory recovery with the use of a conduit may be related to the manner in which conduits help to take tension off of the coaptation and realign nerve ends, especially in the case of size mismatch. Conduits also decrease the incidence of collateral sprouting, permit an environment rich in neurotrophic factors, and protect the coaptation from scar (37). Several additional studies have reproduced similar results and have also demonstrated improvement in recovered in sensation after neurotization in autologous breast reconstruction, strongly suggesting that the sensory recovery of neurotized flaps occurs sooner with improved innervation density that gradually improves over time and has a greater chance of approaching normal sensation compared to a non-innervated flap (Table 1) (35,37-44).
Table 1
| Study | Type of reconstruction | Total number of pts | Number of pts in each group | Number of breasts or flaps in each group | Mean time to follow up in months [range] | Neurotization | Sensory evaluation | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor nerve | Recipient nerve | Technique | Areas tested | Pressure testing | Other testing | ||||||||
| Blondeel (1999), (22) | Unoperated; DIEP–; DIEP+; TRAM– | 104 | 43; 12; 23; 26 | 56; 13; 24; 28 | 19.6 [12–38]; 21.4 [13–40]; 19.9 [12–39] | T10, T11, or T12 | LCB 4th ICN | Direct end to end | Flap skinNative SkinNAC | SWM | Temp, Vib, SEP | DIEP+ flaps had lower pressure thresholds, greater area recovered sensation, higher incidence of erogenous sensation | |
| Speigel (2013), (30) | DIEP–; DIEP+; DIEP+ with NC | 35 | 9; 15; 33 | 111 [23–309] | T11 or T12 | ACB 3rd ICN | Direct end to end | Flap skinNative SkinNAC | PSSD | DIEP+ with NC had lower pressure thresholds than both DIEP+ and and DIEP- | |||
| Slezak (1992), (38) | Unoperated; pTRAM –; pTRAM+ | 23 | 10; 10; 3 | –; –; 6 | 53 [24–84] | T10, T11, or T12 | LCB 4th ICN | Direct end to end | Flap SkinNAC | SWM | 2 pd, Vib | pTRAM+ recovered vibratory sensation earlier | |
| Doncatto (1997), (39) | pTRAM–; pTRAM+ | 54 | 27; 27 | >8 | T11 | LCB 4th, 5th, 6th, or 7th ICN | Direct end to side | finger | Temp, Pain | More pTRAM+ had return of superficial sensation | |||
| Yano (1998), (40) | pTRAM–; pTRAM+ | 31 | 16; 15 | 16; 15 | 24 [11–41]; 14 [4–24]; | T11 or T12 | ACB or LCB or 3rd, 4th, or 5th ICN | Direct end to end | Flap skinNative SkinNAC | SWM | Temp, Pain | TRAM+ had earlier recovery of sensation to touch, pain, and temperature | |
| Yap (2005), (41) | TRAM–; TRAM+ | 14 | 7; 7 | 7; 7 | 40 [31–46]; 39 [35–46] | Single thoraco-abdominal nerve | LCB 4th or 5th ICN | Direct end to end | Flap skinNative SkinNAC | SWM | Temp | TRAM+ had earlier recovery of sensation, lower pressure thresholds, and better recovery of sensation to temperature | |
| Temple (2006), (42) | TRAM–; TRAM+ | 27 | 15; 12 | 19; 18 | 16; 15 | T10 | ACB 4th ICN | Flap skinNAC | SWM | 2 pd, Temp | TRAM+ had lower pressure thresholds, better recovery of sensation to temperature, and a more uniform recovery of sensation throughout the flap | ||
| Puonti (2011), (43) | TRAM–; TRAM+ | 40 | 20; 20 | 20; 20 | 54 [27–77]; 32 [23–43]; | T10, T11, or T12 | Thoraco-dorsal, ICN, or intercosto-brachial nerve | Direct end to end or direct end to side | Flap skinNAC | SWM | Temp, Vib, Pain | TRAM+ had improved total sensory scores | |
| Mori (2011), (44) | pTRAM or pVRAM–; pTRAM or pVRAM+ | 33 | 18; 15 | 18; 15 | [12–57]; [12–19] | T10 or T11 | LCB of 4th ICN | Direct end to end | Flap skinNative SkinNAC | SWM | Temp, Pain | Conventional mastectomy with neurotized flap had lower pressure thresholds and better recovery of sensation to pain | |
DIEP, deep inferior epigastric artery perforator flap; TRAM, transverse rectus abdominis myocutaneous flap; VRAM, vertical rectus abdominis myocutaneous flap; p, pedicled; +, with neurotization; –, without neurotization; NC, nerve conduit. Neurotization; T, thoracic intercostal nerve; ICN, intercostal nerve; LCB, lateral cutaneous branch; ACB, anterior cutaneous branch. Sensory evaluation; NAC, nipple areolar complex; SWM, Semmes-Weinstein monofilament; PSSD, pressure specified sensory device; Temp, temperature; Vib, vibration; SEP, sensory evoked potentials; 2 pd, 2-point discrimination.
Neurotization has been shown not only to improve sensory recovery after autologous breast reconstruction, but to also improve patient rated satisfaction scores. A randomized, prospective study by Temple employed three different assessment tools (the Medical Outcomes Study, the Body Image after Breast Cancer Questionnaire, and the Functional Assessment of Cancer Therapy–Breast quality-of-life instrument) to survey patients about satisfaction in various health related domains following neurotized and non-neurotized TRAM flap breast reconstruction. With the use of the Medical Outcomes Study, patients with neurotized flaps scored higher in six of eight domains, including physical function, physical role, body pain, general health, social function, and emotional role. Similarly, innervated flaps outperformed noninnervated flaps in 4 of the 5 domains within the Body Image after Breast Cancer Questionnaire, including vulnerability, body stigma, limitations, and arm concerns, and outperformed in all 5 of the domains within the Functional Assessment of Cancer Therapy–Breast quality-of-life instrument, which include physical well-being, social well-being, emotional well-being, and functional well-being (13).
Though there is evidence to support that neurotization enhances recovery of sensation, results remain heterogenous and there is little consensus in regards to the optimal technique. Peripheral nerve studies in the upper extremity have shown that repair with nerve allograft results in greater meaningful functional outcomes than repairs with nerve conduits, as defined by at least an M3 or S3 recovery on the Medical Research Council Classification scale, and that outcomes are comparable to repairs with nerve autograft that at shorter lengths, though there is evidence to support their use up to 7 cm (32).There is a paucity of literature surrounding the use of allografts in autologous breast reconstruction, though the anatomical basis for their potential benefit in maximizing sensory recovery is well established (31).
Advancements in microsurgical technique have increased interest and investigation of flap neurotization. Anatomical constraints limiting direct coaptation have led to a variety of techniques to overcome these barriers including long intramuscular dissection of native abdominal intercostal nerves for additional length, use of nerve autograft, and use of nerve allograft (22,29-30). Increased length of donor nerve required for flap neurotization via direct coaptation can be acquired by retrograde dissection through the rectus abdominis fascia and rectus abdominis muscle. This additional dissection, however, increases the risk of motor denervation of the rectus abdominis muscle contributing to abdominal wall donor site morbidity (32-33). Furthermore, the use of the mixed sensory-motor nerves that lie posterior the fascia, as opposed to the use of a purely sensory nerve as is the case once the nerve penetrates the abdominal fascia, introduces multiple blind ends from the severed muscular branches which dilutes the potential directed axonal regeneration and leads to suboptimal sensation recovery (31). Limiting donor nerve dissection and harvest from its exit point from the abdominal wall fascia ensures full potential for sensory regeneration and minimizes the amount of muscular denervation at the expense of nerve length and is the anatomical basis for supporting the use of nerve allografts. The use of nerve allograft compensates for the resulting shorter length of nerve and mimics the revascularization and remodeling that would occur with the use of a nerve autograft but without the morbidities associated with autograft harvesting (32).
Conclusions
Flap neurotization is a microsurgical technique that requires minimal additional operative time and improves both functional outcomes and patient satisfaction following autologous breast reconstruction by enhancing the sensibility of the reconstructed breast. Use of a readily available nerve allograft greatly expands the number of patients who may be able to undergo flap neurotization and benefit from this technique by bridging the anatomical limits from the distance between the recipient to donor nerve. Also, use of nerve allograft decreases the morbidity of secondary sensory loss in those with donor autografts with similar sensory outcomes. Further studies are required to better characterize the role of allograft in augmenting the functional and psychosocial outcomes in autologous breast reconstruction.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dung Nguyen) for the series “Cutting-edge of Complex Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-20-146/coif). The series “Cutting-edge of Complex Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bard M, Sutherland A. Psychological Impact of Cancer And Its Treatment. Cancer 1955;8:656-72. [Crossref] [PubMed]
- Homsy A, Ruegg E, Montandon D, et al. Breast Reconstruction: A Century of Controversies and Progress. Ann Plast Surg 2018;80:457-63. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2017, National Cancer Institute Surveillance, Epidemiology, and End Results Program. Published April 2020. Accessed June 13, 2020. Available online: https://seer.cancer.gov/csr/1975_2017/
- Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203-9. [Crossref] [PubMed]
- O'Halloran N, Potter S, Kerin M, et al. Recent Advances and Future Directions in Postmastectomy Breast Reconstruction. Clin Breast Cancer 2018;18:e571-85. [Crossref] [PubMed]
- Kummerow KL, Du L, Penson DF, et al. Nationwide Trends in Mastectomy for Early-Stage Breast Cancer. JAMA Surg 2015;150:9-16. [Crossref] [PubMed]
- Jonczyk MM, Jean J, Graham R, et al. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res Treat 2019;173:267-74. [Crossref] [PubMed]
- Farhangkhoee H, Matros E, Disa J. Trends and concepts in post-mastectomy breast reconstruction. J Surg Oncol 2016;113:891-4. [Crossref] [PubMed]
- Fancellu A. Considerations arising from requests from patients for a bilateral mastectomy who are eligible for breast-conserving surgery: Factors weighing for and against performing the operation. Oncol Lett 2016;12:764-6. [Crossref] [PubMed]
- Yao K, Sisco M, Bedrosian I. Contralateral prophylactic mastectomy: current perspectives. Int J Womens Health 2016;8:213-23. [Crossref] [PubMed]
- Lazow SP, Riba L, Alapati A, et al. Comparison of breast-conserving therapy vs mastectomy in women under age 40: National trends and potential survival implications. Breast J 2019;25:578-84. [Crossref] [PubMed]
- Shaw WW, Orringer JS, Ko CY, et al. The Spontaneous Return of Sensibility in Breasts Reconstructed with Autologous Tissues. Plast Reconstr Surg 1997;99:394-9. [Crossref] [PubMed]
- Temple CLF, Ross DC, Kim S, et al. Sensibility following Innervated Free TRAM Flap for Breast Reconstruction: Part II. Innervation Improves Patient-Rated Quality of Life. Plast Reconstr Surg 2009;124:1419-25. [Crossref] [PubMed]
- Matthews H, Carroll N, Renshaw D, et al. Predictors of satisfaction and quality of life following post-mastectomy breast reconstruction. Psychooncology 2017;26:1860-5. [Crossref] [PubMed]
- Steffen LE, Johnson A, Levine BJ, et al. Met and Unmet Expectations for Breast Reconstruction in Early Posttreatment Breast Cancer Survivors. Plast Surg Nurs 2017;37:146-53. [Crossref] [PubMed]
- Tsoi B, Ziolkowski NI, Thoma A, et al. Systematic review on the patient-reported outcomes of tissue-expander/implant vs autologous abdominal tissue breast reconstruction in postmastectomy breast cancer patients. J Am Coll Surg 2014;218:1038-48. [Crossref] [PubMed]
- Pusic AL, Matros E, Fine N, et al. Patient reported outcomes 1 year after immediate breast reconstruction: results of the Mastectomy Reconstruction Outcomes Consortium study. J Clin Oncol 2017;35:2499-506. [Crossref] [PubMed]
- Gahm J, Hansson P, Brandberg Y, Wickman M. Breast sensibility after bilateral risk-reducing mastectomy and immediate breast reconstruction: a prospective study. J Plast Reconstr Aesthet Surg 2013;66:1521-7. [Crossref] [PubMed]
- Passavanti MB, Pace MC, Barbarisi A, et al. Pain and sensory dysfunction after breast cancer surgery: neurometer CPT evaluation. Anticancer Res 2006;26:3839-44. [PubMed]
- Aygin D, Cengiz H. Life quality of patients who underwent breast reconstruction after prophylactic mastectomy: systematic review. Breast Cancer 2018;25:497-505. [Crossref] [PubMed]
- Akdeniz Dogan Z, Farhadi J. Evaluation of Sensation on Mastectomy Skin Flaps following Immediate Breast Reconstruction. J Reconstr Microsurg 2020;36:420-5. [Crossref] [PubMed]
- Blondeel PN, Demuynck M, Mete D, et al. Sensory nerve repair in perforator flaps for autologous breast reconstruction: sensational or senseless? Br J Plast Surg 1999;52:37-44. [Crossref] [PubMed]
- Bijkerk E, van Kuijk SMJ, Beugels J, et al. Breast sensibility after mastectomy and implant-based breast reconstruction. Breast Cancer Res Treat 2019;175:369-78. [Crossref] [PubMed]
- Heine N, Koch C, Brebant V, et al. Breast sensitivity after mastectomy and autologous reconstruction. Clin Hemorheol Microcirc 2017;67:459-65. [Crossref] [PubMed]
- Enajat M, Rozen WM, Audolfsson T, et al. Thermal injuries in the insensate deep inferior epigastric artery perforator flap: case series and literature review on mechanisms of injury. Microsurgery 2009;29:214-7. [Crossref] [PubMed]
- Mohanna PN, Raveendran SS, Ross DA, et al. Thermal injuries to autologous breast reconstructions and their donor sites--literature review and report of six cases. J Plast Reconstr Aesthet Surg 2010;63:e255-60. [Crossref] [PubMed]
- Yeniocak A, Kelahmetoglu O, Guneren E. Steam burn as a late complication of nonsensate DIEP flap after breast reconstruction. Microsurgery 2019;39:373-4. [Crossref] [PubMed]
- Nahabedian MY, McGibbon BM. Thermal injuries in autogenous tissue breast reconstruction. Br J Plast Surg 1998;51:599-602. [Crossref] [PubMed]
- Knackstedt R, Grobmyer S, Djohan R. Collaboration between the breast and plastic surgeon in restoring sensation after mastectomy. Breast J 2019;25:1187-91. [Crossref] [PubMed]
- Spiegel AJ, Menn ZK, Eldor L, et al. Breast Reinnervation: DIEP Neurotization Using the Third Anterior Intercostal Nerve. Plast Reconstr Surg Glob Open 2013;1:e72. [Crossref] [PubMed]
- Ducic I, Yoon J, Momeni A, et al. Anatomical Considerations to Optimize Sensory Recovery in Breast Neurotization with Allograft. Plast Reconstr Surg Glob Open 2018;6:e1985. [Crossref] [PubMed]
- Safa B, Buncke G. Autograft Substitutes: Conduits and Processed Nerve Allografts. Hand Clin 2016;32:127-40. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Kiil BJ, et al. Avoiding denervation of rectus abdominis in DIEP flap harvest II: an intraoperative assessment of the nerves to rectus. Plast Reconstr Surg 2008;122:1321-5. [Crossref] [PubMed]
- Ducic I, Safa B, DeVinney E. Refinements of nerve repair with connector-assisted coaptation. Microsurgery 2017;37:256-63. [Crossref] [PubMed]
- Beugels J, Cornelissen AJM, Spiegel AJ, et al. Sensory recovery of the breast after innervated and non-innervated autologous breast reconstructions: A systematic review. J Plast Reconstr Aesthet Surg 2017;70:1229-41. [Crossref] [PubMed]
- Buncke G, Rinker B, Thayer W, et al. Evaluating Nerve Repair Outcomes in Upper Extremity Nerve injuries Utilizing Processed Nerve Allografts, Tube Conduit, and nerve Autograft. J Hand Surg 2015;40:e5. [Crossref]
- Zhou A, Ducic I, Momeni A. Sensory restoration of breast reconstruction – The search for the ideal approach continues. J Surg Oncol 2018;118:780-92. [Crossref] [PubMed]
- Slezak S, McGibbon B, Dellon A. The Sensational Transverse Rectus Abdominis Musculocutaneous (TRAM) Flap: Return of Sensibility after TRAM Breast Reconstruction. Ann Plast Surg 1992;28:210-7. [Crossref] [PubMed]
- Doncatto L, Caleffi M. Breast Reconstruction with Sensitive Tram Flap Reinnervation. Revista Brasileira de Cirurgia Plástica 1997;12:35-46.
- Yano K, Matsuo Y, Hosokawa K. Breast Reconstruction by Means of Innervated Rectus Abdominis Myocutaneous Flap. Plast Reconstr Surg 1998;102:1452-60. [Crossref] [PubMed]
- Yap LH, Whiten SC, Forster A, et al. Sensory Recovery in the Sensate Free Transverse Rectus Abdominis Myocutaneous Flap. Plast Reconstr Surg 2005;115:1280-8. [Crossref] [PubMed]
- Temple CL, Tse R, Bettger-Hahn M, et al. Sensibility following Innervated Free TRAM Flap for Breast Reconstruction. Plast Reconstr Surg 2006;117:2119-27. [Crossref] [PubMed]
- Puonti HK, Jääskeläinen SK, Hallikainen HK, et al. A new approach to microvascular TRAM-flap breast reconstruction – a pilot study. J Plast Reconstr Aesthet Surg 2011;64:346-52. [Crossref] [PubMed]
- Mori H, Okazaki M. Is the sensitivity of skin-sparing mastectomy or nipple-sparing mastectomy superior to conventional mastectomy with innervated flap? Microsurgery 2011;31:428-33. [Crossref] [PubMed]
Cite this article as: Carrau D, Del Pinto Z, Carraher A, Chetta MD. Breast reinnervation—the next frontier in autologous breast reconstruction: a review of early results. Ann Breast Surg 2022;6:14.