Outcomes after skin-reducing mastectomy and immediate hybrid breast reconstruction using combination of acellular dermal matrix and de-epithelialized dermal flap in large and/or ptotic breasts
Introduction
Immediate breast reconstruction following mastectomy has become an integral part of the therapeutic management of breast cancer as well as the prophylactic management of the high-risk breast. Traditionally, implant-based breast reconstruction surgery following a mastectomy was performed by creating a sub-pectoral pocket that provided a total or partial muscle coverage of the prosthetic device (1).
The introduction of acellular dermal matrices (ADMs) and other biological meshes that were used in conjunction with pectoralis major muscle to create a dual-plane reconstruction, showed an improvement in postoperative complications (2). These included better lower pole fullness, more natural ptosis, decreased postoperative pain, and reduction of capsular contracture and implant mispositioning (3,4). A lower implant exchange rate for functional and aesthetic reasons was also found (5).
In recent years pre-pectoral implant-based breast reconstructions incorporating the use of biological meshes have gained in popularity. This technique has become increasingly preferred over the sub-pectoral or dual plane placement of the implant due to several clinical and aesthetic advantages. These included the elimination of window shading, animation deformities, and decreased patient discomfort (6-11). In a select group of patients with small to medium size breasts and little to no ptosis, this procedure has shown to have a low postoperative complication risk (5,6,12-14).
Performing a skin-reducing mastectomy with immediate implant-based breast reconstruction in women with large and/or ptotic breasts is, however, a more challenging task. In this subgroup of patients, a Wise pattern skin-reducing mastectomy is invariably associated with higher complication rates irrespective of the plane of implant placement (14-16). In an attempt to minimize complications associated with sub-pectoral implant reconstruction, Nava and colleagues introduced a novel method of de-epithelializing inferior dermal flap to support the reconstruction and provide the inferior pole cover. This technique enabled surgeons to perform the single-step operation while offering a favorable cosmetic and psychological outcome (16).
In our institution, skin-reducing mastectomy with an immediate prosthetic pre-pectoral reconstruction using de-epithelialized inferior dermal flap in combination with ADM is offered to all women with large and/or ptotic breast. Women with grade three ptosis, a notch to nipple distance of 25 cm or greater, and a breast volume of 700 cc or larger would usually be considered for this procedure. We also offer this procedure to women that have largely fatty involutional breasts, with a smaller breast volume but with a large redundant skin envelope and grade three ptosis. We have adopted the term “hybrid” reconstruction to describe this technique that uses a biologic mesh in conjunction with the classical inferior dermal sling to provide total pre-pectoral implant cover (Figure 1). Currently, there is very little evidence about the safety of the procedure when used in this patient population.
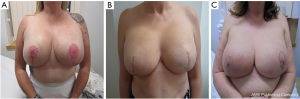
We present the experience of a single institution in performing skin-reducing mastectomies followed by a hybrid reconstruction. We present the following article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-10/rc).
Methods
A single-institution electronic database was used to retrospectively identify patients that underwent a skin-reducing mastectomy and pre-pectoral hybrid breast reconstruction between October 2016 to September 2019. Biologic meshes used in these reconstructions included both porcine and bovine xenografts, more specifically SurgiMend® (Integra LifeScience), Cellis® (Meccellis Biotech), and Meso BioMatrix® (MTF Biologics). Mastectomies were performed for breast cancer and prophylactically in germline genetic mutation carriers. All the mastectomies and immediate hybrid reconstructions were performed by the same two experienced oncoplastic breast surgeons. Both textured permanent expanders with remote ports and textured fixed volume implant-based reconstructions were included in the study. All the implants used were anatomical for a more natural look.
Data including age, body mass index (BMI), mastectomy weight, and risk factors for postoperative complications such as diabetes, active smoking, radiotherapy, and neoadjuvant chemotherapy were collected. Early complications such as infection, tissue necrosis, seroma, hematoma, and implant loss were then analyzed. Time to infection and its influence on the start of the scheduled adjuvant chemotherapy was also collected from the database.
Reconstructions complicated by infection were categorized into major and minor infections, major being those that required a surgical intervention. Minor infections were defined as infections that were successfully treated purely by oral antibiotics.
Tissue necrosis was divided into two groups: (I) full-thickness necrosis, and (II) superficial epidermolysis. The complication of seroma was diagnosed clinically and confirmed by ultrasound. Only those seromas that required an ultrasound-guided aspiration were included in the study. Average follow-up was 31 months (±11.5) from the initial hybrid reconstruction and no patients were lost to follow up.
Statistical analysis
SPSS 25.0 software (IBM Corp., Armonk, NY, USA) was used to complete our statistical analysis. We describe quantitative continuous variables as mean ± standard deviation (SD) and range. One patient’s measurement of implant weight was not available, and this missing data was addressed by reporting valid percent value only.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As advised by London Bridge Research Ethical Committee, no ethical approval was required for this study as this was a retrospective audit with no identifiable patient data. Patient consent was not deemed necessary as we were looking at complications of the technique. We worked with matrices that have been used in our breast unit for the last 6 years, all of which have been Conformité Européenne (CE) marked. Pre-pectoral implant-based hybrid reconstruction using an inferior dermal sling in combination with an ADM is a modification of an existing technique.
Patient selection
We included all patients that underwent a skin-reducing mastectomy and pre-pectoral hybrid reconstruction between October 2016 to September 2019, regardless of their existing co-morbidities, BMI, use of tobacco, neo-adjuvant chemotherapy or radiotherapy, without any exclusions. The operation was offered to breast cancer patients with large and/or ptotic breasts, as well as to those considered at high risk of breast cancer who opted for risk-reducing mastectomies. Our definition of large and ptotic breasts has been addressed in the introduction.
Surgical technique
All the wise pattern skin-reducing mastectomies and hybrid reconstruction using either textured fixed volume anatomical implants or textured permanent anatomical expanders were performed by the same two experienced oncoplastic and reconstructive breast surgeons. The amount of skin excised was dependent on the patients preferred post-operative breast volume. The neo-nipple position was marked at the level of the IMF, the vertical limbs of the wise pattern were then marked by the medial and lateral displacement of the breast around the breast meridian. The skin reduction was in keeping with the desired post mastectomy reconstruction volume. The length of vertical limbs is usually between 6 to 8 cm. Where there is any doubt, we would recommend that one underestimates the amount of skin excised and then trim the skin edges as required at the end of the procedure to ensure a snug but tension free closure. The size of the chosen expanders or implants would depend on the base, height and desired projection of the new reconstructed breast. We were cautious to ensure a tension free closure on every occasion. Permanent anatomical expanders were preferred in smokers to reduce the immediate post-operative bio-mechanical load on the skin flaps. Where solid silicone implants were greater than 650 cc in volume, expanders were occasionally preferred based on skin quality. The decision on using an expander in non-smokers was based on the perceived risk for complications, intended post-reconstruction breast volume, and the quality of skin flaps. Where expanders were used, we ensured that the on-table fill volumes allowed for a tension free closure so as not to overload the skin flaps or compromise the T-junction.
A Wise pattern incision was marked with the patient standing. The mastectomy was performed in a standard fashion after the inferior dermal flap was de-epithelialized and developed. Care was taken to preserve the sub-dermal vascularity of skin flaps by a careful dissection in the anatomical plane at the level of superficial reflection of the superficial fascia. A close clinical evaluation of the skin flap vascularity and viability was performed intraoperatively and was deemed satisfactory in all cases.
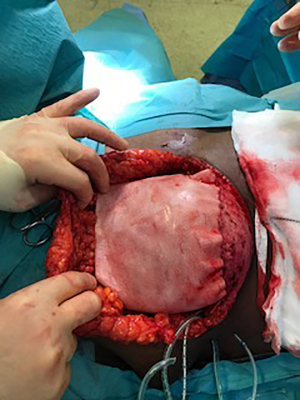
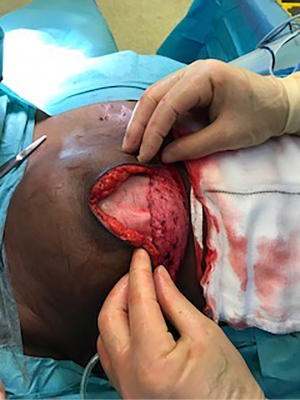
Biological meshes and implants were soaked intra-operatively in an antibiotic solution, containing 1 gm of cefuroxime and 80 mg of gentamycin in one liter of 0.9% normal saline. The biologic mesh was secured to the superior edge of the de-epithelialized dermal flap using a running absorbable monofilament suture, thereby creating one continuous hybrid sheet consisting of the dermal sling inferiorly and the biologic superiorly, that allowed for coverage of the entire anterior surface of the implant (Figure 2). The de-epithelialized dermal flap and matrix were then secured to the chest wall laterally and superiorly with absorbable monofilament interrupted sutures. Two Redivac drains (10 French) were inserted, one in the inferior subcutaneous space and the other in the lateral axillary gutter, and they were secured with a monofilament non-absorbable suture. Tension-free closure was performed over the hybrid reconstruction (Figure 3). The drains were kept in situ until drainage output from each one was less than 30 cc in 24 hours.
Patients stayed in hospital for a maximum of two nights and had their first post-operative review at day 10. Expansion was only started after a minimum of 3 weeks when the wounds had healed completely and there were no concerns about skin viability. The expansion was usually in increments of 150 cc with 2-to-3-week intervals between expansions.
Results
A total of 25 patients (34 breasts) underwent hybrid breast reconstruction after skin-reducing mastectomy over the study period. The patient age ranged from 25 to 74 years, with a median age of 49 (±12.9) years. BMI was greater than 30 kg/m2 in 10 (40%) of our patients placing them in the obese range. Three (12%) patients were active tobacco users who continued smoking after the operation against the surgeon’s advice. Four (16%) patients underwent neoadjuvant chemotherapy and 6 (24%) patients completed radiotherapy (2 patients—neoadjuvant, 4 patients—adjuvant).
The average mastectomy specimen weight was 1,107 (range, 466–2,418) g, with an average implant volume of 568 (range, 420–775) cc. Both, anatomical, textured expander and fixed volume implants were used. Fixed volume implants comprised 76.5% of all reconstructions and expanders were used in 23.5% of all cases (Table 1).
Table 1
| Characteristics | Patients, n=25 (%); breast, n=34 (%) |
|---|---|
| Average age, years (SD) [range] | 49 (±12.9) [25–74] |
| BMI >30 | 10 (40.0) |
| Tobacco use | 3 (12.0) |
| NACT | 4 (16.0) |
| RT | |
| Neoadjuvant | 2 (8.0) |
| Adjuvant | 4 (16.0) |
| Diabetes mellitus | 1 (4.0) |
| ADM | |
| SurgiMend® | 9 (27.3) |
| Cellis® | 17 (50.0) |
| Meso BioMatrix® | 7 (20.6) |
| Expander reconstruction | 8 (23.5) |
| Fixed-volume reconstruction | 26 (76.5) |
| Average mastectomy weight, g (SD) [range] | 1,107 (±538) [466–2,418] |
| Average implant volume cc (SD) [range] | 568 (±90.8) [420–775] |
| Average follow up, months (SD) [range] | 31 (±11.5) [15–57] |
SD, standard deviation; BMI, body mass index; NACT, neoadjuvant chemotherapy; RT, radiotherapy; ADM, acellular dermal matrix.
As summarized in Table 2, major infections requiring re-operation were observed in 4 (11.8%) patients of which 3 (75%) patients were obese with an average BMI of 39±7.8 kg/m2. One of these patients was obese with a BMI of 43 kg/m2 and an active smoker. This reconstruction resulted in implant loss. Three reconstructions were salvaged using a combination of intraoperative washout and negative pressure wound therapy. Amongst the major infections, two involved initial superficial epidermolysis, and one was associated with full-thickness loss at T-junction. The average weight of mastectomy in this group was 1,236 g, and the average time to infection was 29.4±9 days. This postoperative complication did not result in any delay in the initiation of adjuvant chemotherapy.
Table 2
| Complications | Risk factors | ||||||
|---|---|---|---|---|---|---|---|
| N=34 (%) | BMI >30 (%) | Tobacco (%) | NACT (%) | RT | DM | Weight of mastectomy (g) | |
| Major infection | 4 (11.8) | 3 (75.0) | 1 (25.0) | 0 | 0 | 0 | 1,236 |
| Minor infection | 1 (2.9) | 0 | 0 | 1 (100.0) | 0 | 0 | 466 |
| Implant loss | 2 (5.8) | 1 (50.0) | 2 (100.0) | 0 | 0 | 0 | 1,455 |
| Full thickness skin loss | 3 (8.8) | 2 (66.7) | 2 (66.7) | 0 | 0 | 0 | 1,404 |
| Superficial epidermolysis | 6 (17.6) | 3 (50.0) | 0 | 0 | 0 | 0 | 1,035 |
| Seroma | 3 (8.8) | 1 (33.3) | 0 | 0 | 0 | 0 | 638 |
| Hematoma | 1 (2.9) | 1 (100.0) | 0 | 0 | 0 | 0 | 949 |
| Wound dehiscence | 1 (2.9) | 0 | 0 | 1 (100.0) | 0 | 0 | 466 |
BMI, body mass index; NACT, neoadjuvant chemotherapy; RT, radiotherapy; DM, diabetes mellitus.
One patient had a minor infection with superficial wound dehiscence which resolved with a course of oral antibiotics only. This patient had undergone neoadjuvant chemotherapy prior to her surgery.
Implant losses were recorded in 2 (5.8%) patients. One was due to major infection as mentioned previously, and one was a result of full-thickness skin necrosis at T-junction leading to implant exposure. Both patients with implant losses were active smokers.
We observed 3 (8.8%) patients with full-thickness skin necrosis with an average BMI of 41.2 kg/m2, two of which were active smokers with an average mastectomy weight of 1,404 g. Minor superficial epidermolysis was noted in 6 (17.6%) patients all of which healed with no complications.
Other minor complications included 3 (8.8%) seromas requiring ultrasound-guided aspiration, and 1 (2.9%) hematoma. There was no significant difference in complication rate when fixed volume implants were used vs. permanent expanders.
Discussion
Pre-pectoral implant reconstruction is now a well-established technique that is associated with superior clinical and aesthetic outcomes when compared to the classical total sub-pectoral approach. This technique has been shown to avoid complications such as animation deformity, shoulder dysfunction, disruption of pectoral muscle function, and window shading, as well as to decrease postoperative pain (17). Additionally, with the use of ADM, the incidence of capsular contracture in pre-pectoral reconstruction has been reduced due to the lower levels of myofibroblasts in ADM capsules when compared to submuscular capsules (18). Pre-pectoral implant reconstruction is the preferred technique at our institution and is particularly favored in the reconstruction of large and ptotic breasts due to the advantages mentioned earlier. The combination of an ADM and de-epithelialized inferior dermal flap in the pre-pectoral plane defines “hybrid” reconstruction.
To our knowledge, there are only two reported studies that used similar surgical techniques as ours, showing overall complication rates ranging from 9% to 21% (19,20). One of these studies, however, did not include details of the patients' BMI. The second study excludes active tobacco users, morbidly obese patients, and those with co-morbidities.
In our series of 34 hybrid reconstructions, we identified a major infection in 11.8% of patients, of which 75% occurred in morbidly obese patients, with an average BMI of 39 kg/m2. Other complications included superficial and full-thickness necrosis, which were seen at the T-junction in all instances. This is a known vulnerable area in the Wise pattern skin-reducing mastectomies (14-16,21).
We know from the literature that nicotine in cigarette smoke acts as a direct cutaneous vasoconstrictor causing tissue ischemia and impaired healing (22,23). Postoperative complications such as flap necrosis, hematoma, and fat necrosis occur significantly more frequently in smokers than in non-smokers (15,22,23). In line with current reports, we experienced a higher rate of epidermolysis, infection, and implant loss in patients who were obese and active smokers.
Historically, implant-based reconstruction was not recommended in patients with high BMI due to concerns of increased complication rates related to surgery (24), radiation, and chemotherapy (25). Many of these patients who require mastectomy are advised to lose weight before reconstruction can take place.
Although the infection rate is higher in the obese patient population, our study has shown that a high BMI (>30) alone does not exclude patients from being offered immediate implant-based reconstruction. There is concern that obese patients i.e., those with a BMI of greater than 30 kg/m2 are at a high risk of complications and should therefore be offered a delayed reconstruction once they have optimized their BMI. Furthermore, we have demonstrated that this is a safe option in this patient population. However, we have also shown that a combination of high BMI and active smoking poses a great risk for peri-operative complications.
Conclusions
Skin-reducing mastectomy and hybrid breast implant reconstruction is a feasible and relatively safe technique that can be offered to women with large and/or ptotic breasts, that are not morbidly obese and are non-smokers. Bearing in mind that wound dehiscence at the T-junction remains a major cause for concern, this reconstructive technique offers a second vascularized layer of dermis (the dermal sling) between the vulnerable skin flaps and the implant helping prevent implant exposure and consequent loss of reconstruction. Based on our experience, and in keeping with published guidelines and literature, we would strongly recommend that active smokers are counseled on their high risk of peri-operative morbidity and strongly advised to cease the use of all nicotine products for at least 3 weeks before considering an immediate implant reconstruction, to minimize peri-operative surgical site morbidity and implant loss.
Larger comparative studies and additional research is needed to further evaluate early and late complications of hybrid breast reconstruction and overall patient satisfaction.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-10/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-10/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-10/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As advised by London Bridge Research Ethical Committee, no ethical approval was required for this study as this was a retrospective audit with no identifiable patient data. Patient consent was not deemed necessary as we were looking at complications of the technique.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ter Louw RP, Nahabedian MY. Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:51S-9S. [Crossref] [PubMed]
- Wagner RD, Braun TL, Zhu H, et al. A systematic review of complications in prepectoral breast reconstruction. J Plast Reconstr Aesthet Surg 2019;72:1051-9. [Crossref] [PubMed]
- Lee KT, Mun GH. Updated evidence of acellular dermal matrix use for implant-based breast reconstruction: a meta-analysis. Ann Surg Oncol 2016;23:600-10. [Crossref] [PubMed]
- Lardi AM, Ho-Asjoe M, Junge K, et al. Capsular contracture in implant based breast reconstruction-the effect of porcine acellular dermal matrix. Gland Surg 2017;6:49-56. [Crossref] [PubMed]
- Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous direct-to-implant breast reconstruction: surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open 2016;3:e574. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Sbitany H, Langstein HN. Acellular dermal matrix in primary breast reconstruction. Aesthet Surg J 2011;31:30S-7S. [Crossref] [PubMed]
- Salibian AA, Frey JD, Karp NS. Strategies and considerations in selecting between subpectoral and prepectoral breast reconstruction. Gland Surg 2019;8:11-8. [Crossref] [PubMed]
- Nguyen KT, Mioton LM, Smetona JT, et al. Esthetic outcomes of ADM-assisted expander-implant breast reconstruction. Eplasty 2012;12:e58. [PubMed]
- Kankam H, Hourston G, Forouhi P, et al. Combination of acellular dermal matrix with a de-epithelialised dermal flap during skin-reducing mastectomy and immediate breast reconstruction. Ann R Coll Surg Engl 2018;100:e1-6. [Crossref] [PubMed]
- Vidya R, Berna G, Sbitany H, et al. Prepectoral implant-based breast reconstruction: a joint consensus guide from UK, European and USA breast and plastic reconstructive surgeons. Ecancermedicalscience 2019;13:927. [Crossref] [PubMed]
- Vidya R, Iqbal FM. A guide to prepectoral breast reconstruction: a new dimension to implant-based breast reconstruction. Clin Breast Cancer 2017;17:266-71. [Crossref] [PubMed]
- Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: first multicenter European report on 100 cases. Breast J 2017;23:670-6. [Crossref] [PubMed]
- Komorowska-Timek E, Merrifield B, Turfe Z, et al. Subcutaneous prosthetic breast reconstructions following skin reduction mastectomy. Plast Reconstr Surg Glob Open 2019;7:e2078. [Crossref] [PubMed]
- Carlson GW, Bostwick J 3rd, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg 1997;225:570-5; discussion 575-8. [Crossref] [PubMed]
- Nava MB, Cortinovis U, Ottolenghi J, et al. Skin-reducing mastectomy. Plast Reconstr Surg 2006;118:603-10; discussion 611-3. [Crossref] [PubMed]
- Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg 2016;69:e77-86. [Crossref] [PubMed]
- Kim IK, Park SO, Chang H, et al. Inhibition mechanism of acellular dermal matrix on capsule formation in expander-implant breast reconstruction after postmastectomy radiotherapy. Ann Surg Oncol 2018;25:2279-87. [Crossref] [PubMed]
- Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-reduction breast reconstructions with prepectoral implant. Plast Reconstr Surg 2016;137:1702-5. [Crossref] [PubMed]
- Maruccia M, Elia R, Gurrado A, et al. Skin-reducing mastectomy and pre-pectoral breast reconstruction in large ptotic breasts. Aesthetic Plast Surg 2020;44:664-72. [Crossref] [PubMed]
- Derderian CA, Karp NS, Choi M. Wise-pattern breast reconstruction: modification using AlloDerm and a vascularized dermal-subcutaneous pedicle. Ann Plast Surg 2009;62:528-32. [Crossref] [PubMed]
- Vinton AL, Traverso LW, Jolly PC. Wound complications after modified radical mastectomy compared with tylectomy with axillary lymph node dissection. Am J Surg 1991;161:584-8. [Crossref] [PubMed]
- Hwang K, Son JS, Ryu WK. Smoking and flap survival. Plast Surg (Oakv) 2018;26:280-5. [Crossref] [PubMed]
- Panayi AC, Agha RA, Sieber BA, et al. Impact of obesity on outcomes in breast reconstruction: a systematic review and meta-analysis. J Reconstr Microsurg 2018;34:363-75. [Crossref] [PubMed]
- Lee K, Kruper L, Dieli-Conwright CM, et al. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep 2019;21:41. [Crossref] [PubMed]
Cite this article as: Doyle B, Shaari E, Hamed H, Kothari A. Outcomes after skin-reducing mastectomy and immediate hybrid breast reconstruction using combination of acellular dermal matrix and de-epithelialized dermal flap in large and/or ptotic breasts. Ann Breast Surg 2022;6:12.