Incidental mastocytosis in a lady with Cowden syndrome: a case report
Introduction
Hereditary breast cancer is estimated to affect up to 15% of afflicted patients, with more than one first degree relatives with breast cancer(1). Germ line mutations of the BRCA1 and BRCA2 genes account for about 15% of familial breast cancer. Approximately 50% of women with a familial history may also be due to unexplained genes(2). Tumour protein (TP53), cadherin 1 (CDH1), liver kinase B1 (LKB1) and phosphatase and tensin homolog (PTEN) are rarely associated with the development of breast cancer and they account for only about 3% of patients with hereditary breast cancer. Cowden syndrome (CS) is an autosomal dominant inherited cancer syndrome associated with germ line mutations in PTEN, a tumour suppressor gene and is characterized by multiple harmatomas. Patients with CS are at risk of developing malignancies involving breast, thyroid and endometrial(3-5).
Mast cells are associated with benign allergic disorders and malignant conditions namely oesophageal, pancreatic, non-Hodgkin lymphoma and melanoma(6). Some authors hypothesized that mast cells may participate in the inflammatory response by either directly releasing cytokines for tumour angiogenesis or indirectly stimulating inflammatory cells residing in the tumour microenvironment to release more such angiogenic mediators(7). Its exact role in breast cancer remains uncertain. Interestingly, suggestions arise that mast cells may lead to better prognosis though some would disagree(8,9).
We report a case of CS in a young lady who had presented with advanced left breast cancer and was also found to have incidental grade ductal carcinoma in situ (DCIS) on her contralateral side. Our patient happens to be the first with CS to display mastocytosis on pathological examination of both mastectomy specimens. We present the following case in accordance with the CARE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-20-131/rc).
Case presentation
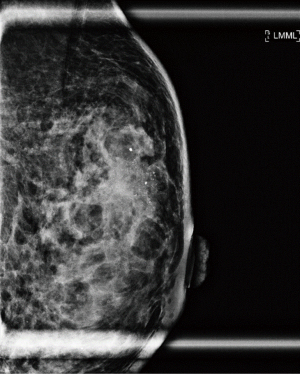
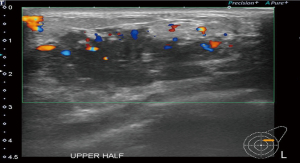
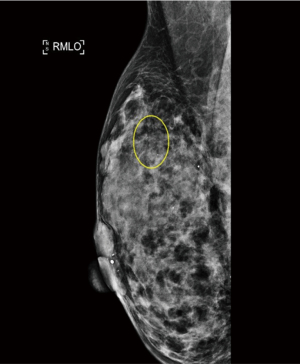
A 37-year-old woman of Asian descent presented with a large left breast mass. Mammogram and ultrasonography suggested that this breast mass was highly suspicious (Figures 1,2). The contralateral breast was screened and a small cluster of amorphous microcalcifications over the upper outer quadrant was observed (Figure 3). Biopsy was performed for both abnormalities. Her left breast mass biopsy confirmed invasive ductal carcinoma (IDC). Estrogen and progesterone receptors were positive and cerB2 equivocal. The subsequent FISH test was negative. Stereotactic sampling of her right breast microcalcifications was atypical ductal hyperplasia (ADH). Computed tomography scan of her thorax, abdomen and pelvis did not show any distant metastasis. Genetic testing was offered in view of her young age and her mother was diagnosed with breast cancer in her early 50s. She was found to have CS. Her head circumference was 60.3 cm, suggestive of macrocephaly and she was observed to have trichilemmomas. She reported to have mild cognitive difficulty and forgetfulness.
Our patient declined endoscopic evaluation of her gastrointestinal tract. She has yet to have a gynaecological evaluation of her endometrium but remains asymptomatic. Her thyroid scan showed multinodular goiter with no compressive symptoms. Fine needle aspiration cytology (FNAC) confirmed Bethesda Category II follicular nodule. No thyroid malignancy was detected.
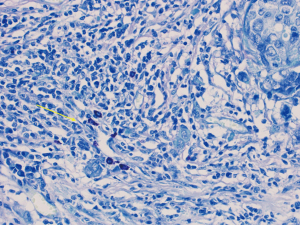
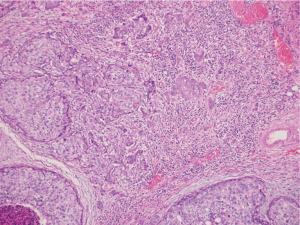
Our patient decided for left mastectomy with prophylactic contralateral mastectomy and bilateral autologous reconstruction at the same setting. The left mastectomy specimen showed Grade 3 IDC measuring 110 mm in size with extensive intraductal component of DCIS. Intraoperative frozen section of the sentinel lymph nodes showed metastatic carcinoma, hence the patient underwent axillary clearance in the same setting. The contralateral prophylactic mastectomy specimen showed low to intermediate grade DCIS at its superior aspect measuring 30 mm in size. No microcalcification was observed in the vicinity of the DCIS, rendering preoperative detection with the aid of mammography to be impossible. The previous biopsy site was not located near the area of the visualised DCIS, indicating that this finding is incidental. An interesting pathological finding on both mastectomy specimens was the increased number of mast cells seen in the stroma of the breast and perilobular areas, which have been reported in some scattered studies involving PTEN associated soft tissue hamartomas (Figures 4,5). We conducted a review of her preoperative biochemical tests, imaging scans and evaluated her postoperative condition. There was no suggestion of systemic mastocytosis. No further treatment was needed for the incidental discovery of mastocytosis. She was assessed to have Stage 3 left breast cancer and Stage 0 right breast cancer. She underwent appropriate adjuvant chemotherapy, radiation and hormonal therapy. No relapse has been detected through clinical examination and biochemical tumour markers during her follow up (Figure 6). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
CS was first described in one Western family in 1963(10). It is an autosomal dominant condition that is linked to germline mutations in the PTEN gene located on chromosome 10q23. A germline mutation in this gene predisposes individuals to benign harmatomatous overgrowth of skin, colon and thyroid and an increased risk of malignancies involving the breast, thyroid and endometrium. Approximately 10–50% of patients have a family history of CS and about 18% do not have any mutations in the PTEN gene(11). Up to 76% of patients with CS are found to have benign breast diseases(12). On the other hand, breast cancers in this group of individuals are often diagnosed at a younger age (38–46 years).
The strength of this study which has yet to be reported in patients with both CS and synchronous bilateral breast cancer is the presence of increased mast cells in both mastectomy specimens. Till date, reports that document mastocytosis in patients with CS only involve soft tissue harmatomas, otherwise known as PTEN harmatoma of soft tissue (PHOST)(13).
Mast cells have traditionally been linked to allergic disorders and are first discovered to exist in tumours by Paul Ehrlich (14). Systemic mastocytosis is a hematological disorder that results in abnormal mast cells proliferation and accumulation. Its clinical manifestations include flushing and hives, gastrointestinal symptoms such as nausea, vomiting and pain, bone and muscle pain and bleeding disorders. Treatment includes identification and avoidance of triggers, medications such as anti histamines or corticosteroids to inhibit further release or chemotherapy or stem cell therapy in cases where systemic mastocytosis are associated with hematological malignancies.
Mast cells comprise of both pro-tumourigenic (e.g., angiogenic and lymphangiogenic) and anti-tumourigenic compounds (e.g., TNF- α and IL-90) (6). Their presence and the inflammatory microenvironment they created is believed to result in cancer development, though the exact role remains largely uncertain. In CS, loss of the PTEN function has been postulated to cause inactivation of the tumour suppressor gene that negatively regulates the phosphatidyl inositol 30 kinase (PI3K)-AKT-mTOR signaling pathway(15). PI3K is counterbalanced by PTEN, which dephosphorylates PIP3. Mutation of PTEN in mast cells does lead to increased levels of PIP3, enhanced cytokine production, proliferation and survival. Furthermore, the mutation of PTEN gene in mice is linked to mastocytosis like disease(16). With the existing molecular studies, we may raise the hypothesis that the PTEN mutation in patients with CS will indirectly lead to mast cells production. Because of its derived factors such as tryptase and histamine, this will in turn be protumourigenic in overall cancer formation and progression(16).
Emerging studies have suggested that mast cells do provoke a worse prognosis in breast cancer patients due to poorer tumour biological features while others disagree on the basis that mast cells were possibly associated with estrogen receptor sensitive tumours (9,17,18). Xiang et al. reported that an increased tryptase leads to higher tumour grade and presence of lymph node metastasis. Coupled with the activation of matrix metalloproteinase-2, the higher levels of tryptase encourage migration of cancer cells along the breast stroma, thereby promoting invasive cancer (19). Another theory is that mast cells might favour lymphangiogenesis and hence, earlier metastasis (7). Initial experimental data with mast cell deficient mice appear to confirm correlation between its presence and mammary cancer. Relevant molecular studies also demonstrated that the release of tryptase in the stromal microenvironment led to its remodeling and myofibroblasts differentiation, hence, contributing to progression of breast cancer (20). One finding observed was the presence of high mast cell density in luminal A and B types or estrogen receptor positive breast tumours (17,18). Such positive correlations suggest that estrogen is a chemotactic agent for mast cells, giving rise to increased proliferation and stimulation of cancer development (21). Our patient had grade 3 tumour over her left breast with lymph node metastasis. Her cancer was hormone receptor positive, suggesting that these features are consistent with observations reported in previous literature.
The limitation of this report remains that this is an isolated and incidental finding of mastocytosis in a single patient with a concomitant diagnosis of CS and bilateral synchronous breast cancer. In spite of the limited data linking mast cells with poor tumour features in breast cancer, the overall role of mast cells and its resultant effect on the prognosis in breast cancer still remain uncertain. No study has yet to document its role in CS induced breast cancer, its clinical effect on tumour biological features and to compare differences with those with non-CS related mammary tumours. Subsequent studies to evaluate this complex molecular pathway that will lead to mammary cancer in CS and ascertain its clinical relevance can be carried out. Consideration can be given to re-examine the mastectomy specimens of affected patients with CS in order to correlate between mast cells and tumour features and evaluate subsequent clinical outcomes.
Conclusions
CS associated breast cancer has been well reported with many cases presenting as bilateral synchronous tumours. Our case is the first to report the presence of mastocytosis in one with CS afflicted with synchronous breast cancer. Molecular studies support our current hypothesis that mastocytosis will affect cancer development, its invasive progression and in turn affect the tumour biological features, even in CS derived breast cancer. More in-depth research to establish this relationship is required.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-20-131/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-20-131/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-20-131/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Collaborative Group on Hormonal Factors in Breast Cancer. Familial Breast Cancer: Collaborative Reanalysis of Individual Data From 52 Epidemiological Studies Including 58,209 Women With Breast Cancer and 101,986 Women Without the Disease. Lancet 2001;358:1389-99. [Crossref] [PubMed]
- Couch FJ, Nathanson KL, Offit K. Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science 2014;343:1466-70. [Crossref] [PubMed]
- Pilarski R. Cowden syndrome: a critical review of the clinical literature. J Genet Couns 2009;18:13-27. [Crossref] [PubMed]
- Zhou XP, Marsh DJ, Morrison CD, et al. Germline inactivation of PTEN and dysregulation of the phosphoinositol-3-kinase/Akt pathway cause human Lhermitte-Duclos disease in adults. Am J Hum Genet 2003;73:1191-8. [Crossref] [PubMed]
- Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract 2010;8:6. [Crossref] [PubMed]
- Varricchi G, Galdiero MR, Loffredo S, et al. Are Mast Cells MASTers in Cancer? Front Immunol 2017;8:424. [Crossref] [PubMed]
- Raica M, Cimpean AM, Ceausu R, et al. Interplay between mast cells and lymphatic vessels in different molecular types of breast cancer. Anticancer Res 2013;33:957-63. [PubMed]
- Mao Y, Keller ET, Garfield DH, et al. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev 2013;32:303-15. [Crossref] [PubMed]
- Glajcar A, Szpor J, Pacek A, et al. The relationship between breast cancer molecular subtypes and mast cell populations in tumor microenvironment. Virchows Arch 2017;470:505-15. [Crossref] [PubMed]
- Lloyd KM 2nd, Dennis M. Cowden's disease. A possible new symptom complex with multiple system involvement. Ann Intern Med 1963;58:136-42. [Crossref] [PubMed]
- Pilarski R, Stephens JA, Noss R, et al. Predicting PTEN mutations: an evaluation of Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome clinical features. J Med Genet 2011;48:505-12. [Crossref] [PubMed]
- Schrager CA, Schneider D, Gruener AC, et al. Clinical and pathological features of breast disease in Cowden's syndrome: an underrecognized syndrome with an increased risk of breast cancer. Hum Pathol 1998;29:47-53. [Crossref] [PubMed]
- Kurek KC, Howard E, Tennant LB, et al. PTEN hamartoma of soft tissue: a distinctive lesion in PTEN syndromes. Am J Surg Pathol 2012;36:671-87. [Crossref] [PubMed]
- Ehrlich P. Beiträge zur Kenntniss der granulirten Bindegewebszellen und der eosinophilen Leukocythen. Arch Anat Physiol 1879;3:166-9.
- Furumoto Y, Charles N, Olivera A, et al. PTEN deficiency in mast cells causes a mastocytosis-like proliferative disease that heightens allergic responses and vascular permeability. Blood 2011;118:5466-75. [Crossref] [PubMed]
- Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 1998;95:29-39. [Crossref] [PubMed]
- Sang J, Yi D, Tang X, et al. The associations between mast cell infiltration, clinical features and molecular types of invasive breast cancer. Oncotarget 2016;7:81661-9. [Crossref] [PubMed]
- della Rovere F, Granata A, Familiari D, et al. Mast cells in invasive ductal breast cancer: different behavior in high and minimum hormone-receptive cancers. Anticancer Res 2007;27:2465-71. [PubMed]
- Xiang M, Gu Y, Zhao F, et al. Mast cell tryptase promotes breast cancer migration and invasion. Oncol Rep 2010;23:615-9. [PubMed]
- Mangia A, Malfettone A, Rossi R, et al. Tissue remodelling in breast cancer: human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology 2011;58:1096-106. [Crossref] [PubMed]
- Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol 2006;102:89-96. [Crossref] [PubMed]
Cite this article as: Lee WP, Shetty SS, Hing JX, Tan CS, Tan SM. Incidental mastocytosis in a lady with Cowden syndrome: a case report. Ann Breast Surg 2022;6:20.