Adjuvant therapy in older patients with breast cancer
Introduction
Japanese women had the highest life expectancy globally in 2020 (87.1 years) (1). Breast cancer is the commonest female cancer, and in Japan, 26.3% of the 95,257 patients diagnosed with this disease in 2016 were >70 years old. Many older adults are diagnosed with cancer and although many die, they also constitute the majority of cancer survivors after treatment. However, the evidence for treating older adults is sparse due to their comorbidities, impaired organ function and frailty, and consequently various options and a decision tree for adequate treatment are needed for them. Clinical trials yield very limited data on which to base guidelines for standard treatment of older breast cancer patients (2). This is mostly because older patients are routinely excluded from, or are at least underrepresented in, clinical trials (3). In oncological practice this can result in frailer older patients receiving less treatment and being subject to more frequent adverse events. This results in worse outcomes despite the administration of standard treatments (4). Consequently, the international standard of care remains undefined. In this context, the American Society of Clinical Oncology (ASCO) issued a Call inviting investigators to assist in developing recommendations for improving the evidence base as a critical need relevant to cancer therapy in older adults (5). To this end, the Japanese Breast Cancer Society (JBCS) launched an annual scientific research assessment in 2018 (4). The International Society of Geriatric Oncology (SIOG) has also published the SIOG 10 priorities initiative to define priorities for the advancement of geriatric oncology in 2011, and recently it has been updated (6). In this review paper, I focus on the current evidence with regard to adjuvant therapy in older patients including Japanese with breast cancer and discuss approaches to making treatment decisions, attitudes toward health-related quality of life (HRQoL), recommendations according to the intrinsic subtypes and how to predict adverse events of chemotherapy.
Approaches to decision making for breast cancer treatment in older patients
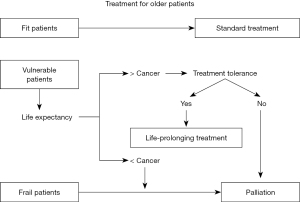
Chronological age is not by itself a robust biomarker and treatments standardized for younger patients should also be offered to older patients deemed sufficiently fit to be able to tolerate it. On the other hand, aggressive treatment might be avoided if the patient would be more likely to die from other causes. The treatment decision tree is shown in Figure 1 according to the reference of the NCCN guideline (2).
First, life expectancy of the older person should be considered to determine appropriate treatments. The estimate of life expectancy in the Japanese population has been provided with a large different range between the upper and lower quartiles (7) and as an online tool ‘ePrognosis’ is available, which is a 10 year-mortality prediction model those was developed and validated in the United States (8). The standard of care depends on the older patient’s estimated life expectancy (9), and of course must always take the patient’s wishes into consideration. On no account should the patient receive suboptimal treatment simply because of age alone. Second, we should consider whether or not breast cancer may affect the patient’s life expectancy. In Japan, 48.0% of deaths of breast cancer patients aged >75 years were not cancer-specific (n=27,385) (4). Thus, life expectancy should be considered as the background to selecting suitable therapies, despite being difficult to predict accurately. In the US, the effect on life expectancy of a diagnosis of breast cancer in older women was investigated using the SEER-Medicare database. This was accomplished by comparing survivals in two groups, one being of 66,000 female breast cancer patients ≥67 years old, and the other being controls matched for age, comorbidity, prior mammography use, and social demographics (10). This study reported the counterintuitive result that women diagnosed with ductal carcinoma in situ (DCIS) or stage I invasive breast cancer actually had a lower risk of death than the controls (HR 0.7, 95% CI: 0.7–0.7 for DCIS: HR 0.8, 95% CI: 0.8–0.8 for stage I breast cancer). In contrast, there was an increased risk of death regardless of age for patients with stage II disease (HR 1.1, 95% CI: 1.0–1.2), and for stage III or IV disease, breast cancer itself was commonest cause of death (10). Third, we should assess the patient’s goals and values regarding the management of the cancer and if they are consistent with anti-cancer treatment. Finally, we consider whether or not the patient can tolerate the standard treatment.
Recommendations for older patients according to the intrinsic subtypes
The human epidermal growth factor receptor 2 (HER2) negative breast cancer (luminal type and triple negative type)
Luminal type breast cancer [Estrogen Receptor (ER)+/HER2–] constitutes 70.0% of the breast cancers in patients >75 years of age, and 64.1% in those aged 55–64 years (4). Triple negative breast cancer (ER–/HER2–) accounts for 14.4% of all breast cancers in patients >75 years old and 13.6% in those aged 55–64 years (4). In randomized controlled trials (RCTs) of older patients, standard cyclophosphamide, methotrexate, and fluorouracil (CMF) or cyclophosphamide plus doxorubicin (AC) chemotherapy regimens were superior to capecitabine, and have been recommended in HER2-negative older breast cancer patients (11,12). In another RCTs, docetaxel plus cyclophosphamide (TC) was superior to AC in older patients as well as younger patients with tolerable adverse profiles, although older patients experienced more febrile neutropenia with TC and more anemia with AC (13). In the retrospective data from the National Cancer Data base indicated that on the effect of adding chemotherapy to local therapy on overall survival in older women with triple negative breast cancer a significant overall survival benefit was observed in favor of administering chemotherapy after adjusting for age, comorbidity score, and tumour factors with propensity-matched analysis (14). It may support consideration of chemotherapy in the treatment of women aged 70 years or older with triple negative breast cancer (14). In another RCTs, docetaxel weekly infusion was not more effective than CMF in older women with breast cancer and even worsens QOL and toxicity (15). In summarize for HER2-negative breast cancer, these data suggest that standard combination regimens are efficacious also in older patients with acceptable toxicity.
HER2-positive breast cancer
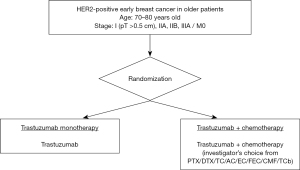
The frequency of such HER2-positive (ER–/HER2+) breast cancers is 5.4% in >75 year-old patients, but >9.8% in those aged 55–64 years (4). The proportion of luminal-HER2 (ER+/HER2+) is 5.3% in patients >75 years old, and >8.6% in those aged 55–64 years in Japan (4). Anti-HER-2 antibody treatments in the adjuvant setting, e.g. trastuzumab (16-19), pertuzumab (20), and trastuzumab emtansine (T-DM1) (21) are standard therapies, but specific data for outcomes of these in older patients are sparse due to their underrepresentation in clinical trials. In addition to chemotherapy, such anti-HER2 targeting therapies are now standard-of-care, but it is still important to demonstrate that they do benefit the older patient, without toxicity caused by chemotherapy. To this end, we conducted an RCT to investigate trastuzumab monotherapy compared with a combination of trastuzumab and chemotherapy in women aged >70 years with HER2-positive primary breast cancer in Japan (RESPECT study, NCT01104935; Figure 2) (22). The null hypothesis was that trastuzumab monotherapy would prove to be non-significantly inferior to trastuzumab plus chemotherapy in terms of disease-free survival, and at the same time superior regarding safety and HRQoL. However, the primary objective of non-inferiority of the monotherapy was not met, although the decreased overall survival without chemotherapy relative to combination therapy was very minor. Hence, it was concluded that in view of the lesser toxicity and improved HRQoL profile, trastuzumab monotherapy can be a viable option for adjuvant therapy in selected older women with breast cancer (23). T-DM1 is an antibody-drug conjugate which combines the HER2-targeting properties of trastuzumab with the cytotoxic activity of the microtubule-inhibitory agent DM1. Tested in the pivotal KATHERINE trial of T-DM1 in the adjuvant setting for HER2-positive early breast cancer patients with residual invasive disease after completion of neoadjuvant therapy, a significantly reduced risk of recurrence or death relative to trastuzumab alone was reported (21). Of note, in the trial among 743 patients registered, only 126 were older than 65 years its hazard ratio was 0.55 (95% CI: 0.22 to 1.34), no definitive result was obtained.
CGA has the potential to predict adverse events of treatment and to identify geriatric problems which reflect supports for standard therapy
Despite the difficulty of distinguishing ‘fit’, from ‘vulnerable’ and ‘frail’ older patients, the aim should be to offer standard treatment to those considered sufficiently fit. To make pre-treatment decisions, CGA should first be performed. This is a cancer treatment planning process following evaluation of the general state and vulnerability of older patients from multiple different perspectives (24). It has a number of multi-disciplinary components, comprising comorbidity, physical function, cognitive function, psychological status, social support care system, nutrition, and medication (25). It may have a potential to predict adverse effects of chemotherapy (26,27) and offers the possibility of identifying potential geriatric problems for which support and additional treatment could be considered (28). The Geriatric-8 screening tool, which includes seven items from the mini-nutritional assessment and age-related items, may be useful for identifying older cancer patients who would benefit from undergoing CGA (24). Recently some tools to predict severe chemotherapy toxicity in older adults with early-stage breast cancer have been developed (29). These tools have been employed to identify patients at risk and to predict their likely prognosis and whether they would be able to tolerate standard therapy. In the opinion of most Japanese physicians, it is useful to include CGA in clinical trials recruiting older patients (30). Indeed, there is an ongoing clinical trial in Japan to test whether CGA can reliably predict adverse events and prognosis (registered ID: UMIN000037454) (31). This has also been included in a phase III study (UMIN000030783) (32).
Considerations regarding HRQoL when making treatment decisions in older patients
In addition to adverse events, HRQoL is important in older patients due to the negative effects of chemotherapy (11). For making appropriate treatment decisions, it is necessary to be aware that older and younger patients do not differ in their acceptance of chemotherapy, but they have different views on balancing survival benefit with immediate QoL impairment (33). That is, older patients may be less willing to trade an incremental absolute survival benefit for a high risk of side effects, loss of independence, and cognitive impairment (34-36). Potentially decreased QOL, even if only temporary, may be a more important consideration of older patients for deciding whether or not to accept chemotherapy (37). In the RESPECT study, detrimental effects of standard adjuvant chemotherapy in older patients, physical and functional well-being, morale, and activity capacity are not transient, but last for at least 12 months (38). In particular during the treatment period, the chemotherapy-induced peripheral neuropathy frequency was high. On the other hand, after 36 months there was no detrimental effects of chemotherapy for any QoL items. It would be possible by adding these explanations to provide relief for patients seeking standard treatment with a combination of chemotherapy and trastuzumab. And also, for at least one year care and social support will be required when adjuvant chemotherapy is given to older patients (38). The European Society for Medical Oncology (ESMO) has proposed a Clinical Benefit Scale incorporating both toxicity and HRQoL as outcomes when assessing the positive and negative impacts of adjuvant therapy (39).
Adverse events of chemotherapy, mainly cardiac toxicity, in older patients
Cardiac toxicity caused by anthracycline-containing chemotherapy in older patients is well-recognized (40,41). Older age and a low left ventricular ejection fraction (LVEF) have been unequivocally established as independent predictors of cardiac side effects (42). Therefore, it is crucial to monitor the occurrence of such of adverse events in older patients. Trastuzumab is also associated with cardiac dysfunction and congestive heart failure (CHF) (43-47). In pivotal studies (19,48-50), the cardiac event rate was highest in the anthracycline-containing trastuzumab patients (1.9–3.8%) and lowest in patients who had received the regimen of docetaxel, carboplatin, and trastuzumab (TCH) (0.4%) (19). As commonly the case, especially for older patients, there is little data on cardiotoxicity of anti-HER2 therapy. Those studies reporting such data are shown in Table 1. However, in the HERA (48), NCCTCN9831 (49), and NSABP B31 (50) trials, only 16% of included patients were >60 years of age, and whether any over 70 were included was not reported. According to a systematic review, cardiac events occurred in 5% of older patients (51) in agreement with a large observational study assessing the risk of cardiotoxicity as 5.7% (52). This could be managed, and was associated with age (52,53). A larger cohort study regarding treatment with trastuzumab in patients over 65 years old has been reported; in 2,203 patients the rate of CHF was found to be 29.4%, whereas this was 18.9% in patients not receiving it (P<0.001). Thus, trastuzumab was more often associated with CHF [hazard ratio (HR), 1.95; 95% CI: 1.75 to 2.17) (54). Of such trastuzumab-treated patients, age >80 years was associated with the occurrence of CHF (HR, 1.53; 95% CI: 1.16 to 2.10), as well as coronary artery disease (HR, 1.82; 95% CI: 1.34 to 2.48), and hypertension (HR, 1.24; 95% CI: 1.02 to 1.50). However, in most cases, cardiotoxicity associated with trastuzumab treatment was reversible (55-59). Thus, it is crucial to start cardiac function monitoring early, considering that most cardiac side effects can be adequately managed (54). Although patient factors predicting the risk and the likelihood of the recovery of LVEF have not been unequivocally identified, troponin I may be such a predictive factor in that patients with higher levels of troponin I are less likely to recover full cardiac function even when provided with heart failure therapy (55). In patients with HER2-positive breast cancer, a number of studies demonstrated value of changes in global longitudinal strain and serum biomarkers, in particular cardiac troponin, in predicting cardiotoxicity (60).
Table 1
| Trial | Treatment ARM | Percentage of older patients | Baseline LVEF (%) | CHF (%) | Cardiac death (n) |
|---|---|---|---|---|---|
| HERA (48) | Chemotherapy→H | >60 y, 16% | ≥55 | 0.6 | 0 |
| Chemotherapy | 0 | 1 | |||
| N9831 (49) | AC→P | >60 y, 16% | ≥50 | 0.2 | 1 |
| AC→PH | 2.5 | 1 | |||
| NSABP B-31 (50) | AC→P | >60 y, 16% | ≥50 | 0.9 | 1 |
| AC→PH | 3.8 | 0 | |||
| BCIRG 006 (19) | AC→D | No information | ≥50 | 0.4 | 0 |
| AC→DH | 1.9 | 0 | |||
| D Carbo H | 0.4 | 0 | |||
| APHINITY (20) | AC→PH/DH; D Carbo H | ≥65 y, 13% | ≥55 | 0.3 | 0 |
| AC→PH/DH/Per; D Carbo H/Per | 0.7 | 0 | |||
| KATHERINE (21) | H | ≥65 y, 8% | >40 | 0.6 | 0 |
| T-DM1 | 0.1 | 0 |
HER2, human epidermal growth factor receptor 2; CHF, congestive heart failure; P, paclitaxel; D, docetaxel; H, trastuzumab; Per, pertuzumab; T-DM1, trastuzumab emtansine.
Pertuzumab is an anti-HER2 humanized monoclonal antibody inhibiting receptor dimerization, a mechanism of action different from trastuzumab. In the APHINITY trial pertuzumab treatment in the adjuvant setting, together with trastuzumab and chemotherapy, led to a significant improvement of invasive disease-free survival of HER2-positive patients with operable breast cancer. However, in that trial, only 13% of participants were >65 years of age (20). The phase II NeoSphere trial investigated combinations of pertuzumab or trastuzumab, or both, with docetaxel in the neoadjuvant setting (61). In the 5-year analysis a slight increase in left ventricular dysfunction occurrence was observed in the pertuzumab group, although it was not statistically different (62). In the pivotal T-DM1 study as an adjuvant setting, KATHERINE trial there was 0.1% cardiac events in the T-DM1 group, 0.6% in the trastuzumab group, respectively, although only 8.5% was older patients (21). In summary with regard to cardiac toxicity, proper monitoring and management of cardiac function are mandatory especially in older patients, the cooperation with cardiologists is important.
Conclusions and perspectives
I reviewed adjuvant therapy in older patients with breast cancer and discussed approaches to decision to treatment, weight of HRQoL, recommendations according to the intrinsic subtypes and adverse events of chemotherapy. The consideration of treatment should be individualized based on health status and patient preference besides anatomical and biological staging, of note, CGA has the potential to identify geriatric problems which reflect support for adequate treatment. It is also important to discuss the balance between survival and quality of life with older patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Masakazu Toi) for the series “Cutting-edge Surgical Research” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-56/coif). The series “Cutting-edge Surgical Research” was commissioned by the editorial office without any funding or sponsorship. MS serves as an unpaid editorial board member of Annals of Breast Surgery from April 2020 to March 2022. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. (2020). World health statistics 2020: monitoring health for the SDGs, sustainable development goals. World Health Organization.
- Older Adult Oncology. NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines (r)). version 1, 2020. 27 February 1 accessed. Available online: https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Sawaki M, Yamada A, Kumamaru H, et al. Clinicopathological characteristics, practical treatments, prognosis, and clinical issues of older breast cancer patients in Japan. Breast Cancer 2021;28:1-8. [Crossref] [PubMed]
- Hurria A, Levit LA, Dale W, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. J Clin Oncol 2015;33:3826-33. [Crossref] [PubMed]
- Extermann M, Brain E, Canin B, et al. Priorities for the global advancement of care for older adults with cancer: an update of the International Society of Geriatric Oncology Priorities Initiative. Lancet Oncol 2021;22:e29-36. [Crossref] [PubMed]
- Iwamoto M, Nakamura F, Higashi T. Estimated life expectancy and risk of death from cancer by quartiles in the older Japanese population: 2010 vital statistics. Cancer Epidemiol 2014;38:511-4. [Crossref] [PubMed]
- ePrognosis. Estimating Prognosis for Elders. 1st May, 2021 accessed. Available online: https://eprognosis.ucsf.edu/
- Wildiers H, Kunkler I, Biganzoli L, et al. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol 2007;8:1101-15. [Crossref] [PubMed]
- Schonberg MA, Marcantonio ER, Ngo L, et al. Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol 2011;29:1570-7. [Crossref] [PubMed]
- Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med 2009;360:2055-65. Erratum in: N Engl J Med 2009;361:1714. [Crossref] [PubMed]
- Muss HB, Polley MC, Berry DA, et al. Randomized Trial of Standard Adjuvant Chemotherapy Regimens Versus Capecitabine in Older Women With Early Breast Cancer: 10-Year Update of the CALGB 49907 Trial. J Clin Oncol 2019;37:2338-48. [Crossref] [PubMed]
- Jones S, Holmes FA, O'Shaughnessy J, et al. Docetaxel With Cyclophosphamide Is Associated With an Overall Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. J Clin Oncol 2009;27:1177-83. [Crossref] [PubMed]
- Crozier JA, Pezzi TA, Hodge C, et al. Addition of chemotherapy to local therapy in women aged 70 years or older with triple-negative breast cancer: a propensity-matched analysis. Lancet Oncol 2020;21:1611-9. [Crossref] [PubMed]
- Perrone F, Nuzzo F, Di Rella F, et al. Weekly docetaxel versus CMF as adjuvant chemotherapy for older women with early breast cancer: final results of the randomized phase III ELDA trial. Ann Oncol 2015;26:675-82. [Crossref] [PubMed]
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84. [Crossref] [PubMed]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659-72. [Crossref] [PubMed]
- Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 2007;369:29-36. [Crossref] [PubMed]
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83. [Crossref] [PubMed]
- von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 2017;377:122-31. [Crossref] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380:617-28. [Crossref] [PubMed]
- Sawaki M, Tokudome N, Mizuno T, et al. Evaluation of Trastuzumab Without Chemotherapy as a Post-operative Adjuvant Therapy in HER2-positive Elderly Breast Cancer Patients: Randomized Controlled Trial Jpn J Clin Oncol 2011;41:709-12. [RESPECT (N-SAS BC07)]. [Crossref] [PubMed]
- Sawaki M, Taira N, Uemura Y, et al. Randomized Controlled Trial of Trastuzumab With or Without Chemotherapy for HER2-Positive Early Breast Cancer in Older Patients. J Clin Oncol 2020;38:3743-52. [Crossref] [PubMed]
- Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018;36:2326-47. [Crossref] [PubMed]
- Taira N, Sawaki M, Takahashi M, et al. Comprehensive geriatric assessment in elderly breast cancer patients. Breast Cancer 2010;17:183-9. [Crossref] [PubMed]
- Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457-65. [Crossref] [PubMed]
- Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol 2016;34:2366-71. [Crossref] [PubMed]
- Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol 2013;24:1306-12. [Crossref] [PubMed]
- Magnuson A, Sedrak MS, Gross CP, et al. Development and Validation of a Risk Tool for Predicting Severe Toxicity in Older Adults Receiving Chemotherapy for Early-Stage Breast Cancer. J Clin Oncol 2021;39:608-18. [Crossref] [PubMed]
- Sawaki M, Tamura K, Shimomura A, et al. ' Choice Practice management for elderly patients with breast cancer; Findings from a survey by the Japan Breast Cancer Study Group. Nagoya J Med Sci 2018;80:217-26. [PubMed]
- Taira N. Observational study on development of screening tool for comprehensive geriatric assessment in elderly breast cancer patients. UMIN 000037454. 16 September 2020, accessed. Available online: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000042693
- A phase III study comparing T-DM1 with pertuzumab, trastuzumab and docetaxel in elderly patients with advanced stage HER2 positive breast cancer (JCOG1607, HERB TEA study). 26, February, 2021 accessed. Available online: https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000034485&language=E.
- Yellen SB, Cella DF, Leslie WT. Age and clinical decision making in oncology patients. J Natl Cancer Inst 1994;86:1766-70. [Crossref] [PubMed]
- Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002;346:1061-6. [Crossref] [PubMed]
- Akishita M, Ishii S, Kojima T, et al. Priorities of health care outcomes for the elderly. J Am Med Dir Assoc 2013;14:479-84. [Crossref] [PubMed]
- Yamaguchi M, Miyashita M, Shimizu C, et al. Factors on decsion towards anti-cancer therapy in advanced or metastatic breast cancer. The 28th Annual Meeting of the Japanese Breast Cancer Society, 2020.
- Kornblith AB, Lan L, Archer L, et al. Quality of Life of Older Patients With Early-Stage Breast Cancer Receiving Adjuvant Chemotherapy: A Companion Study to Cancer and Leukemia Group B 49907. J Clin Oncol 2011;29:1022-8. [Crossref] [PubMed]
- Taira N, Sawaki M, Uemura Y, et al. Health-related quality of life with trastuzumab monotherapy versus trastuzumab plus standard chemotherapy as adjuvant therapy in older patients with HER2-positive breast cancer. J Clin Oncol 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Cherny NI, Sullivan R, Dafni U, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 2015;26:1547-73. [Crossref] [PubMed]
- Pinder MC, Duan Z, Goodwin JS, et al. Congestive Heart Failure in Older Women Treated With Adjuvant Anthracycline Chemotherapy for Breast Cancer. J Clin Oncol 2007;25:3808-15. [Crossref] [PubMed]
- Du XL, Xia R, Liu CC, et al. Cardiac toxicity associated with anthracycline-containing chemotherapy in older women with breast cancer. Cancer 2009;115:5296-308. [Crossref] [PubMed]
- Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol 2010;28:3416-21. [Crossref] [PubMed]
- Cook-Bruns N. Retrospective analysis of the safety of Herceptin immunotherapy in metastatic breast cancer. Oncology 2001;61:58-66. [Crossref] [PubMed]
- Suter TM, Cook-Bruns N, Barton C. Cardiotoxicity associated with trastuzumab (Herceptin) therapy in the treatment of metastatic breast cancer. Breast 2004;13:173-83. [Crossref] [PubMed]
- Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 2007;25:3859-65. [Crossref] [PubMed]
- Ishihara M, Mukai H, Nagai S, et al. Cardiac safety of trastuzumab as adjuvant treatment for Japanese patients with early breast cancer. Int J Clin Oncol 2009;14:431-5. [Crossref] [PubMed]
- Costa RB, Kurra G, Greenberg L, et al. Efficacy and cardiac safety of adjuvant trastuzumab-based chemotherapy regimens for HER2-positive early breast cancer. Ann Oncol 2010;21:2153-60. [Crossref] [PubMed]
- Procter M, Suter TM, de Azambuja E, et al. Longer-Term Assessment of Trastuzumab-Related Cardiac Adverse Events in the Herceptin Adjuvant (HERA) Trial. J Clin Oncol 2010;28:3422-8. [Crossref] [PubMed]
- Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol 2008;26:1231-8. [Crossref] [PubMed]
- Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012;30:3792-9. [Crossref] [PubMed]
- Brollo J, Curigliano G, Disalvatore D, et al. Adjuvant trastuzumab in elderly with HER-2 positive breast cancer: a systematic review of randomized controlled trials. Cancer Treat Rev 2013;39:44-50. [Crossref] [PubMed]
- Dall P, Lenzen G, Göhler T, et al. Trastuzumab in the treatment of elderly patients with early breast cancer: Results from an observational study in Germany. J Geriatr Oncol 2015;6:462-9. [Crossref] [PubMed]
- Vaz-Luis I, Keating NL, Lin NU, et al. Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: a population-based study. J Clin Oncol 2014;32:927-34. [Crossref] [PubMed]
- Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol 2013;31:4222-8. [Crossref] [PubMed]
- Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-Induced Cardiotoxicity: Clinical and Prognostic Implications of Troponin I Evaluation. J Clin Oncol 2010;28:3910-6. [Crossref] [PubMed]
- Morris PG, Hudis CA. Trastuzumab-related cardiotoxicity following anthracycline-based adjuvant chemotherapy: how worried should we be? J Clin Oncol 2010;28:3407-10. [Crossref] [PubMed]
- Telli ML, Hunt SA, Carlson RW, et al. Trastuzumab-Related Cardiotoxicity: Calling Into Question the Concept of Reversibility. J Clin Oncol 2007;25:3525-33. [Crossref] [PubMed]
- Ewer MS. Reversibility of Trastuzumab-Related Cardiotoxicity: New Insights Based on Clinical Course and Response to Medical Treatment. J Clin Oncol 2005;23:7820-6. [Crossref] [PubMed]
- Guarneri V, Lenihan DJ, Valero V, et al. Long-Term Cardiac Tolerability of Trastuzumab in Metastatic Breast Cancer: The M.D. Anderson Cancer Center Experience. J Clin Oncol 2006;24:4107-15. [Crossref] [PubMed]
- Barish R, Lynce F, Unger K, et al. Management of Cardiovascular Disease in Women With Breast Cancer. Circulation 2019;139:1110-20. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791-800. [Crossref] [PubMed]
Cite this article as: Sawaki M. Adjuvant therapy in older patients with breast cancer. Ann Breast Surg 2022;6:15.