
Accelerated partial breast irradiation: current status and future directions
Breast cancer is the most common cancer in women in the USA and globally, and the leading cause of cancer deaths among women globally, while it comes second to lung cancer in the USA (1). Radiation therapy is frequently utilized in the care plan of women with breast and results in less likelihood of local recurrence and possible survival benefit (2). Multiple studies attempted to omit adjuvant radiation therapy in early stage breast cancer; however, there has always been a local control benefit for adjuvant radiation therapy (3-6). APBI has been studied in a number of clinical trials as an alternative to whole breast irradiation in women with early-stage breast cancer with the rationale of decreasing treatment time and volume of tissue irradiated, while maintaining efficacy. Multiple radiation therapy fractionations and modalities have been investigated in order to reach optimal APBI (7-28). Both American Society of Radiation Oncology (ASTRO), and European Society of Radiation Oncology have published consensus APBI guidelines (29,30). In this review, we will present the various APBI approaches. We will focus on external beam radiation therapy (EBRT), brachytherapy (BT), and intra-operative radiation therapy (IORT).
EBRT
EBRT-based APBI does not require special training, unlike BT, nor additional equipment such as intra-operative linear accelerators required for IORT. The majority of women enrolled in APBI clinical trials were treated with EBRT, resulting in a relatively larger clinical experience compared to other APBI approaches. There have been a number of prospective randomized clinical trials that aimed to identify the optimal dose and fraction for EBRT-based APBI. The outcomes of these trials have been inconsistent due to the heterogeneous pool of patients enrolled in these studies, variable EBRT techniques, three-dimensional conformal radiation therapy (3D-CRT) versus Intensity Modulated Radiation Therapy (IMRT), different fractionations, and different methods for volumes delineation (7-11). Some of these clinical trials dictated larger treatment volumes and older 3D-CRT planning which resulted in unfavorable radiation induced toxicity profile and suboptimal cosmetic outcomes compared to conventional breast irradiation (7,8).
The RAPID study (7,8) is a randomized prospective non-inferiority clinical trial that enrolled patients from Canada, Australia and New Zealand, comparing WBI versus APBI utilizing 3D-CRT techniques. The study enrolled ductal carcinoma in-situ (DCIS) or node-negative early stage invasive breast cancer patients who are 40 years of age or older and treated with breast conserving surgery. WBI was delivered either in 42.56 Gy in 16 fractions of 2.66 Gy once daily over 21 days, or 50 Gy in 25 fractions of 2 Gy, once daily over 35 days. Boost radiation to the primary site of 10 Gy in 4–5 fractions once per day was permitted per institutional policy. APBI was delivered in 38.5 Gy in 10 fractions of 3.85 Gy delivered twice daily over 5–8 days. Boost radiation was not permitted in the APBI arm. The study enrolled 2,135 patients, randomized 1:1 to receive APBI or WBI. The 8-year IBTR was 2.8% in the WBI group and 3% in the APBI group (HR 1.27%, 90% CI: 0.84–1.91%). While acute grade ≥2 radiation-induced adverse events favored APBI (28% APBI vs. 45% WBI, P<0.0001), the late grade ≥2 radiation-induced adverse events were more common in APBI (32%) compared to WBI (13%, P<0.0001). Fair or poor cosmesis was also more prevalent in APBI group at 3 years (absolute difference, 11.3%, 95% CI: 7.5–15.0%), 5 years (absolute difference, 16.5%, 95% CI: 12.5–20.4%), and 7 years (absolute difference, 17.7%, 95% CI: 12.9–22.3%) (7, 8).
Meattini et al. (9,10) reported 10-year results of the “FLORENCE study”, a randomized phase III single-institution prospective clinical trial that was conducted at the University of Florence, Italy, between March 2005 and June 2013. The study compared whole breast conventional fractionated tangents fields’ radiation therapy to EBRT-based APBI utilizing IMRT. The investigators screened 5,148 women older than 40 years with early stage breast cancer; 3,702 patients were not eligible for the study, and 926 patients declined participation in the study. A total of 520 patients were randomized to either WBI (260 patients) or APBI (260 patients), in a 1:1 ratio. Exclusion criteria included extensive intra-ductal carcinoma, multifocal breast cancer, and surgical margins less than 5 mm (notably larger margins that current standard of care). APBI was delivered utilizing IMRT technique, 30 Gy in 5 fractions of 6 Gy every other day. The clinical target volume (CTV) was created by 1 cm expansion of the lumpectomy cavity and the surgical clips, and the planning target volume (PTV) was created by a 1 cm of the CTV. At a median follow up of 10.7 years, there was no statistical significant difference in ipsilateral breast tumor recurrence (IBTR) [2.5% in the whole breast irradiation (WBI) group vs. 3.7% in the APBI group, hazard ratio (HR), 1.56; 95% CI: 0.55 to 4.37; P=0.40], the ten-year overall survival (OS) was identical in both groups at 91.9%, the ten-year breast cancer specific survival (BCSS) was 96.7% in the WBI and 97.8% in the APBI arm, and the ten-year distant metastases (DM) was 3.2% in the WBI and 2.8% in the APBI arm. In this study, utilizing IMRT, the cosmetic outcomes favored APBI, which resulted in significantly less radiation therapy induced acute and late toxicity and improved cosmetic outcome as evaluated by both physician and patients (9,10).
NSABP B-39/RTOG 0413 was a prospective randomized, phase 3, equivalence trial in 154 clinical centers in the USA, Canada, Ireland, and Israel, enrolling 4,216 parents between March 21, 2005, and April 16, 2013. The study has the largest, most heterogeneous eligibility criteria including stages 0, I, or II, up to three positive axillary nodes positive, all histologies and multifocal breast cancers, who had lumpectomy with negative surgical margins, which was defined as no detectable cancer cells on inked margins. Patients were randomized to WBRT or APBI. WBRT was delivered in 25 daily fractions of 50 Gy over 5 weeks, with or without a boost to the tumor bed, and APBI was delivered as 34 Gy in 10 fractions using BT or 38.5 Gy in 10 fractions using EBRT, over 5 treatment days within an 8-day period. APBI was delivered utilizing EBRT (73%, 1,536 patients), single entry BT (21%, 451 patients), and multi-catheter BT in (6%, 120 patients). Eighty percent of WBRT patients received an optional 1-week sequential surgical-cavity boost to at least 60 Gy. At a median follow-up of 10.2 years, IBTR was 4% and 3% in APBI and WBRT groups respectively (HR 1.22, 90% CI: 0.94–1.58). The 10-year cumulative incidence of IBTR was 4.6% (95% CI: 3.7–5.7%) in the APBI group versus 3.9% (3.1–5.0%) in the WBRT. Breast Cancer Specific Mortality was 2% in both groups. There was no difference in the second cancers, treatment induced adverse events in both groups, and no treatment related deaths was reported. Regarding the treatment-induced adverse events; grade 1 in 845 (40%), grade 2 in 921 (44%), and grade 3 in 201 (10%) patients in the APBI group, compared with grade 1 in 626 (31%), grade 2 in 1,193 (59%), and grade 3 in 143 (7%) in the WBRT group. The study failed to demonstrate statistical non-inferiority, however, the absolute IBTR difference (0.7%) is arguably not of significant clinical significance, and possibly contributed by the wide range of inclusion criteria, sub-optimal selection of patients for APBI, as they study allowed multi-focal disease (9% of enrolled patients), up to 3 node positive disease (10%), grade 3 disease (26%) and premenopausal women (39%). This is the only large, randomized trial comparing APBI to WBRT, to fail meeting the non-inferiority / equivalence end point (11).
Because cosmetic outcomes of APBI compared to WBRT is an important determinant for treatment method selection, the authors of NSABP-39 performed a subgroup analysis to address the quality of life and cosmesis. The authors found that patient-rated cosmetic outcomes based on global cosmetic score and satisfaction were equivalent for APBI and WBRT groups. However, APBI resulted in worse cosmetic outcome on MD rating. Upon further stratification, the authors found that patients in the APBI group who received chemotherapy and interestingly, patients in the WBRT group who did not receive chemotherapy had worse cosmetic outcomes. The cosmetic outcomes of NSABP 39 differ from the aforementioned RAPID trial which also employed APBI utilizing EBRT. Again, the RAPID trial found late skin toxicity to be 18% worse than conventional WBRT at 7 years. This absolute difference of cosmetic outcomes may be primarily due to an increase in fair cosmesis, because poor cosmesis was uncommon. Ultimately, the optimal dosing and fractionation of EBRT-based APBI remains to be determined to minimize acute and late toxicities and to achieve the best cosmetic outcomes (7,8,11).
MRI-guided APBI
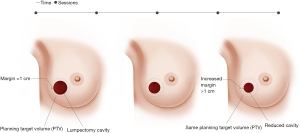
Magnetic resonance image-guided radiotherapy (MRgRT) is an emerging image-guided approach that results in daily optimal visualization and localization of target volumes, which minimize setup uncertainties and enable radiation oncologists to utilize tighter planning target volumes. At Loyola University Chicago, APBI patients undergo both CT and MRI simulation. The MRI images are the primary set of images utilized for radiation planning. Encompassing the lumpectomy cavity, surgical clips and 1 cm margin creates CTV. CTV does not extend into the chest wall muscles and is cropped 5 mm from the skin. PTV is identical to CTV and we do not add any extra margins (Figure 1). The radiation treatment is utilized under MRI guidance and the lumpectomy cavity is used for daily set up and tracking the target, so all MRI-guided APBI treatments are gated, which accounts for intra-fraction motion. We usually treat these patients to 30 Gy in 5 fractions of 6 Gy every other day utilizing free breathing gated MRI-guided IMRT technique (13). We noted significant changes in the size of the lumpectomy cavity over the course of APBI (Figure 2), which raises the question if real-time online adaptive radiation therapy utilizing MRI guidance to redefine the planning target volume prior to every daily treatment is worth investigating. Our group is currently further investigating this preliminary finding.
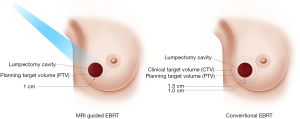
Washington University reported their early results with 30 women treated with MRI-guided APBI utilizing tighter margins of 1 cm universal expansion around the lumpectomy cavity to create the clinical target volume (CTV), with no further expansion to create planning target volume (PTV). Median PTV volume was reduced by 52% when using no PTV margin compared with a 1-cm PTV margin used conventionally. The authors noted minimal intra-fractional motion, and the mean difference between planned and delivered dose was less than 1% (12). The same group recently reported a phase I/II study utilizing MRI-guided APBI that enrolled 50 low-risk, hormone-sensitive breast cancer patients to receive single-fraction, high-gradient partial-breast irradiation 2 to 8 weeks after lumpectomy for node-negative, invasive, or in situ breast cancer. MRI-guided APBI was delivered by prescribing 20 Gy to the surgical bed and 5 Gy to the breast tissue within 1 cm of the surgical bed simultaneously in a single fraction using external beam. The authors reported, at a median follow up of 25 months, no grade 3+ treatment induced adverse events. Good-to-excellent post-treatment cosmesis was present in 100% and 98% per physicians and patients, respectively. One noninvasive in-breast recurrence in a separate untreated quadrant and 1 isolated axillary recurrence, both salvaged successfully. None of the patient had distant recurrences or cancer-related deaths (14).
IORT
IORT involves administering one session of radiation therapy during surgery to the tumor cavity, sparing the patient postoperative EBRT. This is ideal for patients who live a significant distance from a radiotherapy center or may have difficulties undergoing radiation treatment over multiple days. IORT also provides an ideal APBI treatment approach during the current COVID-19 pandemic, allowing patients to avoid postoperative frequent visits to the hospital for adjuvant postoperative radiation treatments. IORT is also thought to inhibit tumor cell repopulation that can occur as oncologists commonly wait 4 weeks for wound closure before delivering radiation therapy. Moreover, an important concern includes a slightly increased risk of death after adjuvant WBRT due to radiation-induced cardiovascular disease (relative risk 1.27; P=0.001) (15-18) and amplification of ischemic heart disease risk by 7.4% per Gy (18).
A study evaluating the effect of surgical wound fluid (WF), collected over 24 h following breast-conserving surgery, on breast cancer cells, found that WF stimulated proliferation, migration, and invasion of breast cancer cell lines. Interestingly the WF stimulatory effect did not exist when they utilized WF from women who received IORT. The WF post-IORT displayed altered proteomic profile and altered expression of several cytokines and failed to stimulate the activation of some intracellular signal transduction pathways, compared to WF from patients who did not receive IORT (19).
The European Institute of Oncology (Milan, Italy) published the results of a prospective randomized study, (ELIOT), in 2013 enrolling women aged 48–75 years with early breast cancer of any histology, with a maximum tumor diameter of 2.5 cm and suitable for breast-conserving therapy were randomly assigned in a 1:1 ratio to receive IORT or EBRT. Patients in the IORT group received one dose of 21 Gy with electrons, to the 90% isodose, to the tumor bed after tumor removal. Those in the EBRT group received 50 Gy in 25 fractions of 2 Gy, followed by a boost of 10 Gy in five fractions. All patients with a positive sentinel lymph node biopsy underwent axillary dissection. In patients with four or more positive axillary nodes, additional EBRT was given as a conventional fractionation of 2 Gy to a total dose of 50 Gy, This was an equivalence trial, and the pre-specified equivalence margin was local recurrence of 7.5% in the IORT group. The primary endpoint was occurrence of IBTR and OS was a secondary outcome. One thousand three hundred and five patients were randomized (651 to IORT and 654 to EBRT) between Nov 20, 2000, and Dec 27, 2007. The 5-year event rate for IBRT was 4.4% in the IORT group and 0.4% in the EBRT group. Nine women (5-year event rate 1.0%) in the IORT and two women (0.3%) in the EBRT developed axillary or other regional lymph node metastasis (P=0.03). Development of contralateral breast cancer was reported in eight patients (1.1%) in the IORT group and in 13 patients (1.7%) in the EBRT group (P=0.34). Development of DM was similar between the two groups (5.1% in IORT vs. 4.8% in EBRT; P=0.94). For the IORT group, 5-year IBTR exceeded 10% in patients with large (>2 cm) tumors, with four or more positive lymph nodes, with poorly differentiated (grade 3) tumors, with estrogen-receptor negative tumors, and with triple-negative breast tumors. In multivariate analysis, these features roughly doubled the risk of IBTR. Overall, 5-year occurrence of IBTR was 11.3% for the 199 women (30.6%) who had at least one of these unfavorable characteristics, but only 1.5% for the remaining 452 women (69.4%; P<0.0001). OS did not differ between the two groups, with a 5-year OS of approximately 97% in both groups. The IORT group reported significantly fewer skin side effects including erythema, dryness, hyperpigmentation, and pruritus. However, there was a higher incidence of fat necrosis in the IORT arm. A subgroup of 178 volunteers (95 from IORT and 83 from EBRT group) underwent follow-up spiral CT. Pulmonary fibrosis was diagnosed in 42 (23.6%) of the patients examined: four (9.5%) underwent IORT and 38 (90.5%) underwent EBRT (P<0.0001), with most cases being grade 1–2 and one being grade 3. In conclusion, the ELIOT trial showed that IORT is equivalent to EBRT as defined by the trial design, though IBTR was significantly worse with IORT compared to EBRT (20).
TARGIT-A trial protocol was based on the observation that most local recurrences occur close to the primary tumor site despite frequent presence of microscopic cancer foci in other quadrants. This prospective, randomized, controlled clinical trial recruited 3,451 women 45 years and older with uni-focal invasive ductal carcinoma up to 3.5 cm in size, cN0-N1, and eligible for breast conservative surgery between 24 March 2000 and 25 June 2012 at 33 centers in 11 countries. Patients were randomized to intraoperative radiotherapy (TARGIT-IORT) (1,721 patients) or EBRT (1,730 patients). TARGIT-IORT was either delivered concurrently with lumpectomy (pre-pathology, n=1,140), or delayed TARGIT (post-pathology, n=581). WBRT was subsequently delivered to patients in the TARGIT-IORT arm if tumor-free margin was less than 1mm, if there were an extensive in situ component (>25%), if unexpected invasive lobular carcinoma was found, or if the patient met other criteria for subsequent WBRT per the discretion of the treatment site. 15.2% of the TARGIT-IORT arm (21.6% pre-pathology, 3.6% post-pathology) received subsequent WBRT because of one or more of these risk factors. The EBRT group received standard fractionated WBRT for three to six weeks of 45–56 Gy, per the treating institution policy. The purpose of the study was to determine whether TARGIT-IORT was non-inferior to EBRT in terms of local recurrence, and also to compare long-term survival outcomes. The non-inferiority margin was specified to be the difference of local recurrence at five years of 2.5% in absolute terms. In the IORT group, the surface of the tumor bed received 20 Gy. At the time of initial results, the 5-year IBTR in the TARGIT-IORT arm was 3.3% (95% CI: 2.1–5.1%) versus 1.3% (0.7–2.5%) for EBRT (P=0.042). Pre-pathology TARGIT-IORT group was non-inferior to the EBRT arm, with 2.1% of patients in the pre-pathology TARGIT-IORT group had IBTR (24/1,140), compared to 1% of the patients in the EBRT arm (11/1,158). However, in post-pathology TARGIT-IORT the between-group difference was larger than 2.5% (5.4% vs. 1.7%; P=0.069). BCSS was 97.4% for TARGIT-IORT arm vs. 98.1%% for EBRT arm (P=0.56). Non-breast cancer specific mortality was significantly lower in TARGIT-IORT arm (1.4%) compared to EBRT arm (3.5%, P=0.0086), and was attributable to fewer deaths from cardiovascular causes and other cancers. The pre-pathology TARGIT-IORT group had 14 fewer deaths (42/1140 vs. 56/1,158) compared with EBRT. OS was 96.1% for TARGIT-IORT arm, compared to 94.7% for EBRT arm (P=0.099). There was no difference in wound related complications but less grade 3 and 4 skin complications in the TARGIT-IORT arm (4 of 1,720 vs. 13 of 1,731, P=0.029) (21).
At a median follow up of 8.6 years (median 8.6 years, maximum 18.90 years, interquartile range, 7.0–10.6), pre-pathology TARGIT-IORT remained non-inferior to EBRT, with no statistical significant difference in IBTR (167 vs. 147 events, P=0.28), mastectomy-free survival (170 vs. 175 events, P=0.74), distant disease- free survival (DMFS) (133 vs. 148 events, P=0.30), OS (110 vs. 131 events, P=0.13), and breast cancer specific mortality (65 vs. 57 events, P=0.54). Non-breast cancer specific mortality remained significantly lower in pre-pathology TARGIT-IORT arm (45 vs. 74 events, P=0.005) compared to EBRT arm (22). Post-pathology TARGIT-IORT long-term follow up data at 9-year follow up met the non-inferiority for IBTR and had no statistical significant difference in DMFS and OS (23).
BT
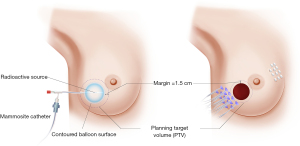
BT can be delivered through single-catheter, or multiple catheters high dose rate (HDR) BT machines (Figure 3), or through permanent seed implants low dose rate (LDR) radioactive sources. While BT has always resulted in favorable cosmetic outcomes, and comparable local control, it requires advanced training, special expertise, and access to LDR radioactive sources or HDR BT machines.
Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO) conducted a phase III, randomized controlled multi-center trial between April 20, 2004 and July 30, 2009. Patients were randomized to APBI using interstitial multicatheter BT (n=633) and WBRT with tumor bed boost (n=551). Patients were considered eligible if they were 40 years or older, had pTis or pT1-2a (lesions of
Polgár et al. reported the 20-year data of a Hungarian study for 258 women with early stage breast cancer who underwent breast conservative surgery, and were randomized to APBI utilizing interstitial multi-catheter high-dose-rate (HDR) BT APBI with 7×5.2 Gy b.i.d. or 50 Gy EBRT versus WBRT. The 20-year results confirmed their previously published result; there were no significant differences in the ipsilateral breast tumor recurrences rates (9.6% vs. 7.9%; P=0.59), disease-free (79.7% vs. 78.3%), cancer-specific (92.6% vs. 88.1%), or overall survival (59.5% vs. 59.7%) in APBI and WBRT arms, respectively. There were significantly better cosmetic outcomes (excellent or good) in the APBI utilizing BT compared to WBRT groups (79.2% vs. 59.5%; P=0.0007). The authors concluded that interstitial HDR BT significantly improved cosmetic results compared to WBRT (26-28).
Conclusions
Thousands of women with early stage breast cancer were enrolled in prospective randomized clinical trials comparing various APBI techniques versus conventional WBRT and the results have been reported. The majority of these trials demonstrated statistical non-inferiority of APBI compared to WBRT, with the exception of NSABP B-39/RTOG 0413, likely due to the wide range of eligibility criteria in this study, however, the IBTR difference was less than 1% between APBI and WBRT in NSABP B-39/RTOG 0413 (7-12,20-28). While APBI utilizing BT has been superior to the older EBRT based APBI and resulted in favorable cosmetic outcomes compared to WBRT, the recent EBRT based APBI utilizing modern planning techniques and IORT results are promising. Patients’ selection in compliance of the published guidelines is of ultimate importance to avoid treatment failures (29,30). BT requires advanced training, expertise, and availability of BT machines. IORT long-term published data is strong and provides a convenient and efficacious APBI modality when offered to appropriate patients’ populations. There is ample evidence to support APBI for appropriately selected women with early stage breast cancer based on the available level 1 evidence as discussed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Breast Surgery for the series “Advancements and Opportunities for Breast Irradiation”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-20-151/coif). The series “Advancements and Opportunities for Breast Irradiation” was commissioned by the editorial office without any funding or sponsorship. WS Jr served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Breast Surgery from August 2019 to July 2021. WS Jr reports honoraria for invited talks from Carl Zeiss, Advisory Board August 2019 from Varian, Advisory Board January 2019 from Merck, Co-Chair of NRG Gyn Committee until June 2020, receiving salary support forwarded to his institution from NRG Oncology, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Blamey RW, Bates T, Chetty U, et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer 2013;49:2294-302. [Crossref] [PubMed]
- Kunkler IH, Williams LJ, Jack WJL, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266-73. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343 J Clin Oncol 2013;31:2382-7. [Crossref] [PubMed]
- Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 2004;351:963-70. [Crossref] [PubMed]
- Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol 2013;31:4038-45. [Crossref] [PubMed]
- Whelan TJ, Julian JA, Berrang TS, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet 2019;394:2165-72. [Crossref] [PubMed]
- Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 2015;51:451-63. [Crossref] [PubMed]
- Meattini I, Marrazzo L, Saieva C, et al. Accelerated Partial-Breast Irradiation Compared With Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial. J Clin Oncol 2020;38:4175-83. [Crossref] [PubMed]
- Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet 2019;394:2155-64. [Crossref] [PubMed]
- Acharya S, Fischer-Valuck BW, et al. Magnetic Resonance Image Guided Radiation Therapy for External Beam Accelerated Partial-Breast Irradiation: Evaluation of Delivered Dose and Intrafractional Cavity Motion. Int J Radiat Oncol Biol Phys 2016;96:785-92. [Crossref] [PubMed]
- Omari E, Gambla J, Mai H, et al. Implementing a real-time magnetic resonance imaging guided accelerated partial breast irradiation program [abstract]. In: Proceedings of the 2019 San Antonio Breast Cancer Symposium; 2019 Dec 10-14; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2020;80:Abstract nr P4-12-21.
- Kennedy WR, Thomas MA, Stanley JA, et al. Single-Institution Phase 1/2 Prospective Clinical Trial of Single-Fraction, High-Gradient Adjuvant Partial-Breast Irradiation for Hormone Sensitive Stage 0-I Breast Cancer. Int J Radiat Oncol Biol Phys 2020;107:344-52. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr 2010;2010:162-77. [PubMed]
- Lee LJ, Harris JR. Innovations in radiation therapy (RT) for breast cancer. Breast 2009;18:S103-11. [Crossref] [PubMed]
- Belletti B, Vaidya JS, D'Andrea S, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 2008;14:1325-32. [Crossref] [PubMed]
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [Crossref] [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer:5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [Crossref] [PubMed]
- Vaidya JS, Bulsara M, Baum M, et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ 2020;370:m2836. [Crossref] [PubMed]
- Vaidya JS, Bulsara M, Saunders C, et al. Effect of Delayed Targeted Intraoperative Radiotherapy vs Whole-Breast Radiotherapy on Local Recurrence and Survival: Long-term Results from the TARGIT-A Randomized Clinical Trial in Early Breast Cancer. JAMA Oncol 2020;6:e200249. [Crossref] [PubMed]
- Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet 2016;387:229-38. [Crossref] [PubMed]
- Polgár C, Ott OJ, Hildebrandt G, et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:259-68. [Crossref] [PubMed]
- Polgár C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma—5-year results of a randomized trial. Int J Radiat Oncol Biol Phys 2007;69:694-702. [Crossref] [PubMed]
- Polgár C, Fodor J, Major T, et al. Breast-conserving therapy with partial or whole breast irradiation: ten-year results of the Budapest randomized trial. Radiother Oncol 2013;108:197-202. [Crossref] [PubMed]
- Polgár C, Major T, Takácsi-Nagy Z, et al. Breast-Conserving Surgery Followed by Partial or Whole Breast Irradiation: Twenty-Year Results of a Phase 3 Clinical Study. Int J Radiat Oncol Biol Phys 2021;109:998-1006. [Crossref] [PubMed]
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated Partial Breast Irradiation Consensus Statement From the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987-1001. [Crossref] [PubMed]
- Polgár C, Limbergen E, Van , Pötter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: Recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol 2010;94:264-73. [Crossref] [PubMed]
Cite this article as: Refaat T, Chan DP, Kamel S, Thomas T, Hentz C, Vaince F, Godellas CV, Small W Jr. Accelerated partial breast irradiation: current status and future directions. Ann Breast Surg 2022;6:16.

