
Bowen’s disease of the nipple: a case report
Introduction
Bowen’s disease is a plaque-like, erythematous lesion that symbolizes a low grade, intraepithelial variant of squamous cell carcinoma (1). Growth of this tumor is intraepidermal and its progression and history is similar to other intradermal carcinomas. These lesions usually appear on the trunk, lower extremities, and sun exposed areas such as the head and neck (2). However, they can appear on other areas of the body as rarely seen here on the nipple. It can present, as it did in this case, with a circumscribed scaly appearance that is usually erythematous and could weep. Progression of the lesion is usually slow and can extend over years. Invasive cancer can arise from these lesions but metastasis from this invasive component is rare (3). Upon diagnosis surgical excision with clear margins should be performed. We report our experience with an extremely rare case of Bowen’s disease of the nipple. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/abs-20-154).
Case presentation
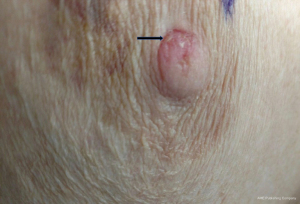
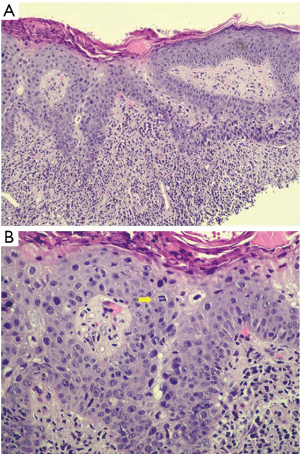
A 73-year-old female was referred to the breast clinic due to scaling, crusting, scabbing, and irritation on her left nipple of 3–4 months duration. She reported no family history of breast cancer and denied trauma to the area. She denied pain and drainage from the breast. Her last screening mammogram was 9 months prior and it was read as BIRADS category 2, benign. Prior to referral to breast surgery, she had been treated with topical steroid cream for the past 3–4 months without resolution. On physical examination, there was scaling and irritation of the left nipple (Figure 1). No palpable masses in the breast and no axillary or cervical lymphadenopathy were identified. The contralateral breast was normal. Diagnostic mammography did not reveal any abnormality in the nipple or retroareolar region and it was reported as benign. An ultrasound of the breast was unremarkable. She underwent a punch biopsy of the lesion, which revealed SCCIS with lichenoid inflammation and no evidence of Paget’s disease. The decision was made to proceed with wide local excision of the left nipple and carrying the incision through full thickness of the skin into the subcutaneous fatty tissue. No glandular breast tissue was excised. There were no surgical complications. Final pathology revealed focal SCCIS with no evidence of invasive disease and clear margins (Figure 2).This patient has remained disease free at 22 months. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Since its first documentation in 1912 by J.T Bowen as a cutaneous chronic atypical epithelial proliferation, Bowen’s disease has since been better classified as a cutaneous squamous cell carcinoma in situ (SCCIS) (1). This nomenclature emphasizes the restriction of the lesion to the epidermis without evidence of dermal invasion (4). The commonest sites of occurrence are the head, neck and sun exposed areas of torso and extremities. Very rarely does the disease occur in non-sun-exposed areas hence the rarity of Bowen’s disease of the nipple as is the case of this patient. The first reported case of Bowen’s disease of the nipple was documented by Cremer in 1982 (5) and to the best of our knowledge, only 10 cases have been reported to date, with four of those cases reported in North America in Liang et al.’s study (6). This further stresses the importance and rarity of our patient’s disease.
Typically, Bowen’s disease of the nipple presents as a gradual enlargement of a well-demarcated erythematous epidermal plaque associated with hyperkeratosis, pruritus, inflammation, and desquamation of the skin (7). Less commonly it can occur as papules, plaques, or nodules which may be smooth, hyperkeratotic or ulcerated. Our patient had the usual symptoms of an itchy scaly lesion. This presentation poses a diagnostic challenge to breast oncologists since other dermal pathologies such as Paget’s disease, melanoma and eczema present with similar symptoms. It is no surprise; therefore, that histologic examination is an integral component of diagnosis.
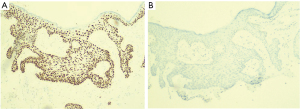
Histopathological features consistent with Bowen’s disease include abnormal mitoses of the epidermis, presence of dyskeratosis, and proliferation of atypical cells that do not exhibit evidence of dermal invasion (3). While useful, this does not clearly differentiate Bowen’s disease of the nipple from other intraepidermal malignancies of the nipple-areola complex such as Paget’s disease that is the most common. This differentiation is usually achieved by immunohistochemical staining. Paget cells stain positively for CK7, CEA, CAM5.2, GCDFP-15 while Bowen’s disease stain for CK5/6, p63, and 34BE-12 (Figure 3A) and are usually negative for CK7 (6,8) (Figure 3B). This finding is crucial in confirming the diagnosis and was consistent with the staining results obtained in the index patient.
Although the rarity of this disease in the nipple has limited an in-depth understanding of its risk factors, however, general risk factors for Bowen’s disease include sunlight exposure, human papillomavirus, immunosuppression, arsenic exposure, and chronic inflammation. The patient discussed above, however, had none of these predisposing factors. Genetic mutations in exons of the CDKN2A locus and RAS pathways have been linked to cutaneous squamous cell carcinoma but their role in Bowen’s disease is not clear (9). At this juncture, it is pertinent to note that the progression of Bowen’s disease of the nipple to invasive squamous cell carcinoma is low, ranging between 3–9% (2).
Multiple treatment modalities have been proposed, including surgical and non-surgical options. Conservative, non-surgical treatments include cryotherapy with liquid nitrogen, curettage with cautery, topical fluorouracil, cryotherapy, excision, imiquimod, photodynamic therapy, topical diclofenac (10). While these modalities lead to an ablation of the cells, there is concern for spread via lactiferous ducts, likely due to continuation of the nipple epidermal layer. This implies that some residual disease might be left untreated. This has led to the adoption of wide local excision as the gold standard of treatment, hence the rationale for the treatment choice for this patient (2,3,11). All the documented cases of Bowen’s disease of the nipple in the literature have shown no recurrence. Our patient likewise has remained with no evidence of disease. In conclusion, we report a rare case of Bowen’s disease of the nipple managed successfully by wide local excision.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-154
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-154). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bowen JT. Centennial paper. May 1912 (J Cutan Dis Syph 1912;30:241-255). Precancerous dermatoses: a study of two cases of chronic atypical epithelial proliferation. By John T. Bowen, M.D., Boston. Arch Dermatol 1983;119:243-60. [Crossref] [PubMed]
- Kitahara M, Hozumi Y, Watanabe A, et al. Bowen's Disease of the Nipple. Case Rep Oncol 2018;11:609-14. [Crossref] [PubMed]
- Sharma R, Iyer M. Bowen's disease of the nipple in a young man with AIDS: a case report. Clin Breast Cancer 2009;9:53-5. [Crossref] [PubMed]
- Brookes PT, Jhawar S, Hinton CP, et al. Bowen's disease of the nipple-a new method of treatment. Breast 2005;14:65-7. [Crossref] [PubMed]
- Cremer H, Paulussen F. Bowen's disease of the nipple. Geburtshilfe Frauenheilkd 1982;42:590-2. [Crossref] [PubMed]
- Liang DG, Soliman B, Cha J. A rare case of Bowen's disease of the nipple: Literature review and management pathway. Breast J 2020;26:1234-8. [Crossref] [PubMed]
- Blobstein SH, Wolfin NS, Urmacher C, et al. Pagetoid Bowen's disease on the breast. Int J Dermatol 1986;25:381-2. [Crossref] [PubMed]
- Ishikawa M, Ohtsuka M, Yamamoto T. Bowen's Disease of the Nipple and Areola in an Old Man. Indian J Dermatol 2015;60:424. [Crossref] [PubMed]
- Hosaka N, Uesaka K, Takaki T, et al. Poorly differentiated squamous cell carcinoma of the nipple: a unique case for marked exophytic growth, but little invasion with neuroendocrine differentiation. Med Mol Morphol 2011;44:174-8. [Crossref] [PubMed]
- Cox NH, Eedy DJ, Morton CA, et al. Guidelines for management of Bowen's disease: 2006 update. Br J Dermatol 2007;156:11-21. [Crossref] [PubMed]
- Venkataseshan VS, Budd DC, Un Kim D, et al. Intraepidermal squamous carcinoma (Bowen's disease) of the nipple. Hum Pathol 1994;25:1371-4. [Crossref] [PubMed]
Cite this article as: Torabi M, Onyemkpa CJ, Samat S, Tan Y, Soleimani T, Thrasher M, Bumpers HL. Bowen’s disease of the nipple: a case report. Ann Breast Surg 2021;5:41.

