Hybrid breast reconstruction: a systematic review of current trends and future directions
Introduction
Breast reconstruction is known to substantially improve patient-reported outcomes after mastectomy (1). The two primary types of post-mastectomy breast reconstruction utilize either autologous or implant-based techniques. A third option, however, is hybrid breast reconstruction (2). Hybrid breast reconstruction involves the simultaneous use of both implants and autologous tissue to recreate the breast mound. In patients with limited options for autologous reconstruction, hybrid techniques can allow for restoration of a more natural breast shape, especially when compared to the use of prosthetic reconstruction alone (3).
In women with limited availability of vascularized donor tissue, hybrid breast reconstruction can help overcome limitations of autologous reconstruction alone. In such patients, hybrid breast reconstruction provides a means by which to safely and reproducibly achieve adequate breast volumes with natural contour, by supplementing autologous tissue with an implant. While other autologous solutions have been proposed for use in such women, including stacked flaps and secondary fat grafting, these options are either technically challenging or inconsistent with regards to achieving large breast volumes (3). Hybrid breast reconstruction also presents advantages over traditional alloplastic breast reconstruction (4). Use of a flap to supplement soft tissue coverage of an implant can improve aesthetic outcomes and minimize risk of contour deformities/rippling, especially when considering prepectoral implants. Furthermore, flap coverage can minimize negative consequences of irradiation on the implant.
Though preliminary reports of hybrid breast reconstruction are from the 1990s, there has been a resurgence of literature on this technique (5). In fact, improved patient selection and perioperative management in recent years have enhanced outcomes of this breast reconstruction modality (6). However, there are currently no systematic reviews on hybrid breast reconstruction, to help summarize such improvements in practice. Thus, the objective of this paper was to systematically review the current literature on hybrid breast reconstruction, in order to synthesize recent innovations in the field and explore areas for further research. We present the following article in accordance with the PRISMA reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-20-114/rc).
Methods
This was a systematic review of the English-language literature on hybrid breast reconstruction. A structured literature search was performed using PubMed, MEDLINE, EMBASE, Scopus, the Cochrane Central Register of Controlled Trials, the New York Academy of Medicine Grey Literature Report, the World Health Organization Library Database, and Web of Science with the MeSH terms listed in Appendix 1. Covidence software (Melbourne, Australia) was utilized to manage study screening, quality assessment, and data extraction. Study selection was guided by predefined inclusion criteria, formulated using a Population, Intervention, Comparison, Outcome, Timing, and Setting (PICOTS) framework. Inclusion criteria for the articles were as following: (I) focus on hybrid breast reconstruction (e.g., patient selection, operative techniques, and postoperative clinical or patient-reported outcomes); (II) original scientific article (i.e., not a review article or meta-analysis); and (III) English-language article published since 2000. The year 2000 was selected given that this review was focused on synthesizing the most current literature on the topic. An updated search was conducted during the review process (November 2020), to enhance the currency of the systematic review.
PRISMA guidelines were used to manage the study workflow (7). Two independent investigators screened titles, abstracts, and full texts of articles that were identified through the literature search process. Only articles whose primary objectives were to describe techniques for or investigate the outcomes of hybrid breast reconstruction were selected. Discrepancies were resolved through consensus. Reviewers assessed risk of bias for each study included in the final cohort using the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) scale (8). The authors extracted information on study objectives, design, interventions, results, and conclusions. The reference sections of articles that were selected for the final study cohort were also reviewed to identify any further relevant articles.
Results
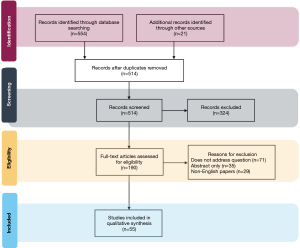
In total, 575 English language articles were identified from the initial query, of which 55 articles were selected for inclusion in the final review. Figure 1 is a flow diagram demonstrating selection of the final set of literature reviewed for this study.
Most included studies employed observational cohort study designs or were case series, while some were clinical trials or conference papers. Overall risk of bias was low for 45 studies and moderate for 10 studies (Figure S1). All articles provided details about surgical techniques and assessed postoperative complications after breast reconstruction. Thirty-five studies also provided data on patient-reported outcomes and 27 studies provided information on indications for hybrid reconstruction as well as patient selection.
Discussion
From review of large database studies and national registries, hybrid procedures have been reported to comprise anywhere from under 5% to over 20% of post-mastectomy breast reconstructions (9,10). Preoperative, intraoperative, and postoperative considerations for hybrid breast reconstruction synthesized from the literature are summarized below.
Indications and patient selection
Multiple indications for hybrid breast reconstruction have been reported in the literature. One of the most commonly-cited indications is lack of sufficient donor site tissue to achieve desired breast volumes (11). This can occur when there is a mismatch between desired breast size and available tissue at the donor site (i.e., thin body habitus with medium to large, ptotic breasts), especially in cases where bilateral reconstruction is required (12,13). In fact, studies of hybrid breast reconstruction have demonstrated that women undergoing this type of breast reconstruction had a lower mean BMI than those undergoing autologous reconstruction (3). This can also occur when women have undergone prior abdominal procedures or do not want extensive abdominal surgery, thereby limiting use of this donor site for large reconstructions.
In the aforementioned population, another important indication for using hybrid breast reconstruction over alloplastic reconstruction alone is neoadjuvant or adjuvant radiotherapy. Traditionally, autologous reconstruction is preferred if radiotherapy is required. This presents a challenge in women who do not have sufficient donor tissue to undergo autologous reconstruction. Hybrid reconstructive techniques can allow women to achieve desired breast sizes with cosmetically-acceptable outcomes even in the setting of breast irradiation.
Overall, appropriate patient selection is key to the success of hybrid breast reconstruction. The volume of tissue available at the chosen donor site is not paramount, given that the hybrid technique allows for volume augmentation with an implant. Rather, appropriate adipofascial laxity at the selected donor site is considered to be one of the most important characteristics for successful hybrid breast reconstruction, to allow for a low-tension closure (11). In fact, Kanchwala and Momeni (2018) described an algorithm for hybrid breast reconstruction: if there is sufficient adipofascial laxity in the absence of sufficient volume at the desired donor site, the patient is a candidate for hybrid breast reconstruction. The hybrid reconstruction should be completed prior to any radiotherapy: flap coverage of the implant is protective and minimizes risks of capsular contracture or implant exposure (3).
Operative considerations
Review of the literature has highlighted multiple important technical considerations with regards to hybrid breast reconstruction. These include: (I) timing of the reconstruction (e.g., immediate, delayed-immediate with tissue expander placement, or delayed) and of implant placement (e.g., concurrently with the flap or delayed after the flap), (II) anatomic plane of implant placement (e.g., prepectoral versus dual-plane/subpectoral), and (III) type of implant (e.g., silicone versus saline).
With regards to timing of the reconstruction, immediate, delayed-immediate and delayed reconstructive techniques have all been successfully described in the literature. However, each technique has its respective indications and limitations (Table 1) (14,15). For instance, while some studies report concurrent implant placement with flap reconstruction, others advocate for delayed implant placement to protect the vascular pedicle of the flap (16,17). In the literature, delayed implant placement has been associated with a lower rate of long-term implant-related complications, as well as a lower rate of implant revisions/exchanges (18).
Table 1
| Indications/advantages | Limitations | References | |
|---|---|---|---|
| Timing of reconstruction/implant | (14-18) | ||
| Immediate | ● Immediate volume enhancement | ● Risk of implant-induced pedicle compression | |
| ● Single-stage reconstruction | ● Risk of implant migration | ||
| ● Implant size is limited by both the delicate mastectomy skin and flap pedicle, as well as by swelling of the flap in the immediate postoperative setting, which may necessitate future size adjustments | |||
| Delayed-immediate | ● Enhanced control of pocket size: expansion of skin pocket can accommodate larger implants | ● Requires multiple operations | |
| ● Implant can be placed after irradiation to minimize risks of radiotherapy | ● Requires multiple clinic visits for tissue expansion | ||
| ● Moderate risk for pedicle compression | |||
| Delayed | ● Lowest risk for immediate pedicle compression | ● Requires multiple operations | |
| ● Allows for best assessment of implant size necessary to achieve satisfactory breast volumes | ● Pocket dissection can be technically challenging due to scar tissue/fibrosis | ||
| ● Pocket size is limited by degree of existing skin laxity | |||
| Anatomic plane of implant | (3,4,12,19,20) | ||
| Subpectoral | ● Minimal risk of pedicle impingement | ● Risk for animation deformity | |
| ● Risk for bottoming-out of implant over time | |||
| Prepectoral—ADM Pocket | ● Moderate risk of pedicle injury—ADM can be used to protect the vascular pedicle | ● Increased cost due to use of ADM | |
| ● Allows for better control of pocket and can re-use pocket | ● Theoretical risk for seromas secondary to ADM use | ||
| ● ADM helps define the inframammary fold and provides inferior support to the implant, reducing pressure on overlying flap and minimizing risk for bottoming-out of implant | |||
| Prepectoral—subcutaneous pocket | ● Less expensive and less technically demanding than creation of an ADM pocket | ● Risk for bottoming-out of implant over time | |
| ● Highest risk of pedicle injury | |||
| Implant type | (12,21-23) | ||
| Saline | ● Easier to recognize implant rupture | ● Implant rippling is often more noticeable | |
| ● Can allow for postoperative volume adjustments via remote injection port | ● More firm than native breast tissue | ||
| Silicone | ● Cohesive gel implants better recapitulate native breast tissue | ● Greater risk for silent rupture | |
| ● Less visible/palpable rippling |
ADM, acellular dermal matrix.
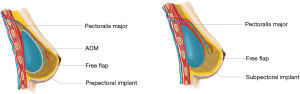
The anatomic plane in which the implant is placed requires careful consideration when free flaps are used for hybrid reconstruction, given the risk of the implant impinging on the vascular pedicle (Table 1; Figure 2). Multiple studies have described the creation of a submuscular pocket using the pectoralis major and serratus anterior, to secure the implant and create a protective layer of muscle between the implant and the vascular pedicle (19). When transverse rectus abdominis myocutaneous (TRAM) flaps are used, the rectus abdominis muscles can also contribute to muscular coverage of the implant (12). Prepectoral implant placement in hybrid breast reconstruction is also gaining popularity, and it is especially preferred in women with irradiated breasts (20).
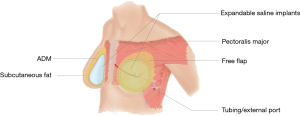
Both silicone and saline implants have been used in hybrid breast reconstruction (Table 1) (12,21,22). The authors have also recently reported the successful use of adjustable saline implants with hybrid reconstructive techniques (Figure 3). Adjustable saline implants were demonstrated to enhance patient satisfaction by allowing for postoperative modifications of breast volume (Zhou A, Yesantharao P, Nguyen D, unpublished data).
Outcomes of hybrid reconstruction, by flap type
Multiple flap options for hybrid breast reconstruction have been described in the literature (Table 2) (9,23). With regards to microvascular reconstruction, successful use of both internal mammary and axillary recipient vessels for hybrid reconstruction has been reported in the literature (3,12).
Table 2
| Indications/advantages | Limitations | References | |
|---|---|---|---|
| Pedicled flaps | |||
| Latissimus dorsi (LD) myocutaneous flap | ● Versatile flap with multiple variations (FELD, scar-less, etc.) that can be used when abdominally-based flaps are contraindicated | ● Risk for LD atrophy and implant exposure, especially with denervated flaps | (12,20,24) |
| ● Quicker operation and faster postoperative recovery than abdominally-based flaps | ● Risk for loss of shoulder force/function | ||
| ● Muscular tissue does not recapitulate the natural breast contour as well as abdominally-based flaps | |||
| ● Less vascularized tissue coverage of implant than abdominally-based flaps- may necessitate larger implant volumes | |||
| Thoracodorsal artery perforator flap |
● Spares the LD muscle | ● May require LD muscle if skin paddle is not robust | (25-29) |
| Inframammary adipofascial flap | ● Avoids an additional incision | ● Limited volume available for reconstruction | (30) |
| Transverse rectus abdominis musculocutaneous flap | ● Avoids functional limitations associated with LD flaps | ● Abdominal donor-site morbidity, including risk for abdominal hernias | (12,13) |
| ● Can improve abdominal contour | ● Can be contraindicated in women with prior abdominal surgery | ||
| ● More accurately recreates the natural breast contour | |||
| ● Lower rates of flap necrosis compared to LD flaps due to smaller implant requirements | |||
| ● Lower rates of capsular contracture than LD flaps due to more robust vascularized tissue coverage of implant | |||
| Free flaps | |||
| Deep inferior epigastric perforator flap |
● Avoids functional limitations associated with LD flaps | ● Risk for abdominal donor site morbidity | (3,6,31,32) |
| ● Can improve abdominal contour | ● Can be contraindicated in women with prior abdominal surgery | ||
| ● More accurately recreate the natural breast contour | ● Technically more challenging than pedicled TRAM | ||
| ● Preserves abdominal musculature unlike TRAM flap | ● Highest risk of vascular compromise | ||
| Transverse upper gracilis flap | ● Can be used in patients without adequate adipofascial laxity of the abdomen | ● Less tissue available than abdominal donor site | (33) |
Latissimus dorsi (LD) myocutaneous flap
The LD is a workhorse flap for hybrid breast reconstruction that is reported to safely and reliably provide well-vascularized muscular coverage for implants, even in the setting of radiotherapy (1,34-38). This technique for hybrid reconstruction is versatile, with reported uses ranging from salvage procedures to reconstructive surgeries for large, ptotic breasts (39-43). Multiple variations on this flap have been reported, including fat-enriched LD (FELD) flaps as well as scar-less LDs, which are raised without creating donor site scars (44-46). Overall, the implant plays a greater role than the flap in determining final breast volume in hybrid reconstructions with LD flaps (24,47-49). This can result in greater need for downstream implant revisions (50).
There is debate in the literature about whether the thoracodorsal nerve should be sectioned when using the LD for implant coverage in hybrid breast reconstruction. This is done to avoid unintentional postoperative movements of the LD muscle with shoulder activation, resulting in animation deformity of the underlying implant. However, flap denervation poses a risk for LD atrophy and implant exposure, which can compromise reconstructive and aesthetic outcomes (12,51).
Patient reported outcomes with regards to LD flap-based hybrid reconstruction demonstrate mixed results (52). Some studies report high patient satisfaction (25,53-55). In fact, one study of irradiated patients reported a mean overall satisfaction score of 8.78 out of 10, while another reported that 80% would undergo the same operation again (23,50). However, other studies have demonstrated no improvements in BREAST-Q scores when hybrid LD flaps with implants were compared to LD flaps alone (26,47).
Thoracodorsal artery perforator (TDAP) flap
TDAP flaps (also known as TAP flaps) can reduce donor site morbidity when compared to traditional musculocutaneous LD flaps for hybrid breast reconstruction (27,28). These flaps often spare the LD muscle, but require an implant to achieve adequate breast volumes (29,30). With regards to operative technique, a skin flap is harvested based on a perforator derived from the descending branch of the thoracodorsal vessels. The flap is then pedicled anteriorly and secured to the thoracic wall, after which a tissue expander or implant is placed. In some studies, a portion of the LD has also been harvested and used with the TDAP flap to provide additional cushioning for the perforator-implant interface (31).
Inframammary adipofascial flap
Hybrid reconstruction with inframammary adipofascial flaps has been reported in the literature as a way to avoid the extra incision and donor site morbidity associated with LD or TDAP flap-based hybrid techniques (32). This technique involves using an adipofascial flap pulled up through the inframammary mastectomy incision, thereby reducing donor site morbidity. One case report of this technique in the literature demonstrated good cosmetic reconstruction of moderately-ptotic breasts upon long-term follow up.
Transverse rectus abdominis musculocutaneous (TRAM) flap
Both free and pedicled TRAM flaps have been used for hybrid breast reconstruction (13). As with other abdominally-based flaps, final breast volumes in TRAM flap-based hybrid reconstruction are largely dictated by the flap rather than the implant, given that a greater degree of autologous tissue is available (12). This facilitates use of smaller implants, thereby reducing pressure on the overlying flap and mastectomy skin and minimizing risk of necrosis (12). When compared to autologous TRAM flap reconstruction alone, hybrid techniques may result in lower rates of donor site morbidity because smaller volumes of tissue are harvested from the donor site, resulting in a lower-tension abdominal closure.
Deep inferior epigastric perforator (DIEP) flap
DIEP flaps also have a long history of use in hybrid breast reconstruction (33). As with TRAM flaps, the advantages of DIEP flaps include abdominal contouring and the ability to use smaller implants given that the DIEP flap can contribute a substantial amount of volume to the breast reconstruction (3). However, considering that flap volume is augmented with an implant, only excess tissue is harvested from the donor site and a lower, more aesthetically-pleasing scar can be utilized than when DIEP flaps are used alone.
Overall complication rates of hybrid reconstruction with DIEP flaps have been reported to be lower on average when compared to hybrid reconstruction with other flap types (6). No cases of flap loss after DIEP flap-based hybrid reconstruction have been reported in the literature (3). However, fat necrosis has been reported in patients who underwent post-mastectomy radiotherapy, and thus some authors propose the use of TRAM flaps for hybrid reconstruction if irradiation is anticipated (3). Overall, the literature has reported good aesthetic outcomes after hybrid reconstruction with DIEP flaps (6,56).
Transverse upper gracilis (TUG) flap
In patients who do not have adequate adipofascial laxity of the abdomen but who desire hybrid breast reconstruction, the TUG flap may provide an appropriate alternative. A case report of TUG reconstruction with implants demonstrated that this technique allowed for adequate restoration of preoperative breast volumes with no postoperative complications reported in either the donor or recipient site (57). Further investigation of this technique is necessary, as an alternative to hybrid reconstruction with abdominally-based free flaps.
Hybrid breast reconstruction versus other reconstructive techniques
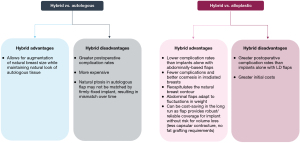
Hybrid breast reconstruction has been demonstrated to have various advantages and disadvantages when compared to either alloplastic or autologous techniques alone, in terms of both clinical outcomes and costs (Figure 4) (12,58,59). In patients with irradiated breasts, for instance, hybrid breast reconstruction has been demonstrated to have a clear benefit when compared to implant-based reconstruction alone (60). In irradiated breasts, placement of a flap over the implant may protect the implant from the negative consequences of radiotherapy. The vascularized flap tissue creates a pocket for the implant and minimizes direct contact between the implant and irradiated tissue. This may enhance wound healing and protect against capsular contracture, infection, as well as wound breakdown. Thus, though autologous reconstruction is traditionally recommended in women with irradiated breasts, hybrid reconstruction may provide a reasonable alternative in patients who require the extra volume provided by an implant. In a series of 1,000 irradiated breasts, hybrid breast reconstruction with either LD or free TRAM flaps was found to result in significantly lower rates of implant loss (5% versus 30.3%) and reconstructive failure (15.2% or 10.0% versus 42.2%, respectively) than implant-only reconstructions (20).
Future directions
Hybrid reconstruction has evolved out of a need to improve clinical and cosmetic outcomes of breast reconstruction in patients with inadequate donor site volumes. In fact, enhancing natural reconstruction with implants can achieve results comparable to cosmetic surgery, thereby helping to satisfy patients’ expectations with regards to the cosmesis of their reconstructed breasts (3). More formal assessments of patient-reported outcomes with validated measures will help to better understand the utility of hybrid breast reconstruction. Future work should also focus on further minimizing postoperative complications and enhancing aesthetic outcomes of hybrid techniques. For instance, the authors have recently described the use of adjustable saline implants with abdominally-based free flaps. By facilitating postoperative modifications of implant fill volumes, this technique was used to optimize final breast size without the need for additional surgery (Zhou A, Yesantharao P, Nguyen D, unpublished data). Furthermore, use of the inflatable saline implant allowed for controlled pocket size expansion without compromising the flap/pedicle. In fact, adjustable saline implants were associated with significantly lower rates of postoperative implant revisions, and they helped to improve overall patient satisfaction (Zhou A, Yesantharao P, Nguyen D, unpublished data). As such, it is possible that these adjustable implants can be used with any flap type to further improve the hybrid breast reconstruction process. Further investigation is warranted.
Study limitations
This study was not without limitations. First, the search strategy may not have comprehensively captured all relevant articles. However, we surveyed multiple databases including those specific to grey literature, and we updated the search during the review process to ensure that the captured articles were comprehensive and relevant. Second, there is the risk of reporting bias, given the systematic review study design. However, information from each study was extracted by two independent reviewers, to help minimize the risk of such bias.
Conclusions
This article reviewed indications, techniques, and outcomes of hybrid breast reconstruction. Multiple options for hybrid reconstruction have been described in the literature, each with unique indications and limitations. Hybrid techniques are indicated for postmastectomy breast reconstruction in patients who lack sufficient donor site tissue to recreate their desired breast volumes, especially when breast irradiation is anticipated. Hybrid breast reconstruction has been demonstrated to successfully recapitulate natural breast shapes and volumes, without incurring excessive postoperative risks of implant or flap loss. Furthermore, hybrid reconstruction avoids technical complexities of techniques such as stacked flap reconstruction to achieve larger breast volumes when donor site tissue is limited. Overall, hybrid breast reconstruction allows for autologous reconstruction even in the setting of insufficient donor site tissue volumes, and can help to improve patients’ satisfaction with the final outcomes of their breast reconstruction procedures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Breast Surgery for the series “Cutting-edge of Complex Breast Reconstruction”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-20-114/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-20-114/coif). The series “Cutting-edge of Complex Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. DHN served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Breast Surgery from November 2019 to December 2021. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Laporta R, Sorotos M, Longo B, et al. Tips and Tricks to Improve Clinical and Aesthetic Outcomes in Latissimus Dorsi Flap Breast Reconstruction. J Reconstr Microsurg 2017;33:455-65. [Crossref] [PubMed]
- Momeni A, Kanchwala S. Delayed-immediate hybrid breast reconstruction-Increasing patient input and precision in breast reconstruction. Breast J 2019;25:898-902. [Crossref] [PubMed]
- Kanchwala S, Momeni A. Hybrid breast reconstruction—the best of both worlds. Gland Surg 2019;8:82-9. [Crossref] [PubMed]
- Momeni A, Kanchwala SK. Improved pocket control in immediate microsurgical breast reconstruction with simultaneous implant placement through the use of mesh. Microsurgery 2018;38:450-7. [Crossref] [PubMed]
- Evans GR, Schusterman MA, Kroll SS, et al. Reconstruction and the radiated breast: is there a role for implants? Plast Reconstr Surg 1995;96:1111-5; discussion 1116. [Crossref] [PubMed]
- Walters JA 3rd, Sato EA, Martinez CA, et al. Delayed Mammoplasty with Silicone Gel Implants following DIEP Flap Breast Reconstruction. Plast Reconstr Surg Glob Open 2015;3:e540. [Crossref] [PubMed]
- Welch V, Petticrew M, Tugwell P, et al. PRISMA-Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med 2012;9:e1001333. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Casella D, Calabrese C, Orzalesi L, et al. Current trends and outcomes of breast reconstruction following nipple-sparing mastectomy: results from a national multicentric registry with 1006 cases over a 6-year period. Breast Cancer 2017;24:451-7. [Crossref] [PubMed]
- Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol 2011;18:1665-70. [Crossref] [PubMed]
- Momeni A, Kanchwala S. Reply: Hybrid Prepectoral Breast Reconstruction: A Surgical Approach That Combines the Benefits of Autologous and Implant-Based Reconstruction. Plast Reconstr Surg 2019;144:319e-20e. [Crossref] [PubMed]
- Kronowitz SJ, Robb GL, Youssef A, et al. Optimizing Autologous Breast Reconstruction in Thin Patients. Plast Reconstr Surg 2003;112:1768-78. [Crossref] [PubMed]
- Serletti JM, Moran SL. The Combined Use of the TRAM and Expanders/Implants in Breast Reconstruction. Ann Plast Surg 1998;40:510-4. [Crossref] [PubMed]
- Bittar SM, Sisto J, Gill K. Single-stage breast reconstruction with the anterior approach latissimus dorsi flap and permanent implants. Plast Reconstr Surg 2012;129:1062-70. [Crossref] [PubMed]
- Lee HC, Han HH, Kim EK. Use of a Vertical Muscle-Sparing Latissimus Dorsi Flap in Implant-Based Breast Reconstruction Without Position Change. Ann Plast Surg 2018;81:152-5. [Crossref] [PubMed]
- Feng J, Pardoe CI, Mota AM, et al. Two-Stage Latissimus Dorsi Flap with Implant for Unilateral Breast Reconstruction: Getting the Size Right. Arch Plast Surg 2016;43:197-203. [Crossref] [PubMed]
- Bach AD, Morgenstern IH, Horch RE. Secondary "Hybrid Reconstruction" Concept with Silicone Implants After Autologous Breast Reconstruction - Is It Safe and Reasonable? Med Sci Monit 2020;26:e921329. [Crossref] [PubMed]
- Roehl KR, Baumann DP, Chevray PM, et al. Evaluation of outcomes in breast reconstructions combining lower abdominal free flaps and permanent implants. Plast Reconstr Surg 2010;126:349-57. [Crossref] [PubMed]
- Miller MJ, Rock CS, Robb GL. Aesthetic Breast Reconstruction Using a Combination of Free Transverse Rectus Abdominis Musculocutaneous Flaps and Breast Implants. Ann Plast Surg 1996;37:258-64. [Crossref] [PubMed]
- Chang DW, Barnea Y, Robb GL. Effects of an Autologous Flap Combined with an Implant for Breast Reconstruction: An Evaluation of 1000 Consecutive Reconstructions of Previously Irradiated Breasts. Plast Reconstr Surg 2008;122:356-62. [Crossref] [PubMed]
- Pacella SJ, Vogel JE, Locke MB, et al. Aesthetic and technical refinements in latissimus dorsi implant breast reconstruction: a 15-year experience. Aesthet Surg J 2011;31:190-9. [Crossref] [PubMed]
- McCarthy CM, Klassen AF, Cano SJ, et al. Patient satisfaction with postmastectomy breast reconstruction: a comparison of saline and silicone implants. Cancer 2010;116:5584-91. [Crossref] [PubMed]
- Tarantino I, Banic A, Fischer T. Evaluation of Late Results in Breast Reconstruction by Latissimus Dorsi Flap and Prosthesis Implantation. Plast Reconstr Surg 2006;117:1387-94. [Crossref] [PubMed]
- He WY, El Eter L, Yesantharao P, et al. Comparing Clinical Outcomes For TRAM, DIEP, And Latissimus Dorsi Flap Breast Reconstructions: A Systematic Review And Meta-Analysis. Toronto, Canada: Plastic Surgery Research Council, 2020.
- Venus MR, Prinsloo DJ. Immediate breast reconstruction with latissimus dorsi flap and implant: audit of outcomes and patient satisfaction survey. J Plast Reconstr Aesthet Surg. 2010;63:101-5. [Crossref] [PubMed]
- Winters ZE, Afzal M, Balta V, et al. Patient-reported outcomes and their predictors at 2- and 3-year follow-up after immediate latissimus dorsi breast reconstruction and adjuvant treatment. Br J Surg 2016;103:524-36. [Crossref] [PubMed]
- Hamdi M, Salgarello M, Barone-Adesi L, et al. Use of the Thoracodorsal Artery Perforator (TDAP) Flap With Implant in Breast Reconstruction. Ann Plast Surg 2008;61:143-6. [Crossref] [PubMed]
- Børsen-Koch M, Gunnarsson GL, Udesen A, et al. Direct delayed breast reconstruction with TAP flap, implant and acellular dermal matrix (TAPIA). J Plast Reconstr Aesthet Surg 2015;68:815-21. [Crossref] [PubMed]
- Brackley PT, Mishra A, Sigaroudina M, et al. Modified muscle sparing latissimus dorsi with implant for total breast reconstruction - extending the boundaries. J Plast Reconstr Aesthet Surg 2010;63:1495-502. [Crossref] [PubMed]
- Lorenzen MM, Gunnarsson GL, Bille C, et al. Visualized bilateral breast reconstruction by propeller thoracodorsal artery perforator flaps. Gland Surg 2019;8:S262-70. [Crossref] [PubMed]
- Bank J, Ledbetter K, Song DH. Use of thoracodorsal artery perforator flaps to enhance outcomes in alloplastic breast reconstruction. Plast Reconstr Surg Glob Open 2014;2:e140. [Crossref] [PubMed]
- Ogawa T, Yamakawa T. A case report of total breast reconstruction using an inframammary adipofascial flap with an implant. Int J Surg Case Rep 2016;23:109-11. [Crossref] [PubMed]
- Rodebeck EM, Blum CA, DellaCroce FJ. Stacked DIEP and Implant for Unilateral Breast Reconstruction. J Reconstr Microsurg Open 2017;2:e124-5. [Crossref]
- DeLong MR, Tandon VJ, Rudkin GH, et al. Latissimus Dorsi Flap Breast Reconstruction—A Nationwide Inpatient Sample Review. Ann Plast Surg 2017;78:S185-8. [Crossref] [PubMed]
- Garusi C, Lohsiriwat V, Brenelli F, et al. The value of latissimus dorsi flap with implant reconstruction for total mastectomy after conservative breast cancer surgery recurrence. Breast 2011;20:141-4. [Crossref] [PubMed]
- Hardwicke JT, Prinsloo DJ. An analysis of 277 consecutive latissimus dorsi breast reconstructions: a focus on capsular contracture. Plast Reconstr Surg 2011;128:63-70. [Crossref] [PubMed]
- Dutra AK, Junior JA, Fernandes ACN. Delayed breast reconstruction with transverse latissimus dorsi myocutaneous flap using Becker expander implants in patients submitted to radiotherapy: A series of cases. J Plast Reconstr Aesthet Surg 2019;72:1067-74. [Crossref] [PubMed]
- Chiasson KF, Kumbla PA, Restrepo RD, et al. Immediate Latissimus Dorsi and Prosthetic Reconstruction in the Setting of Postmastectomy Radiation: An Analysis of 376 Breast Reconstructions. Ann Plast Surg 2020;84:S364-8. [Crossref] [PubMed]
- Kokosis G, Khavanin N, Nahabedian MY. Latissimus Dorsi Musculocutaneous Flap for Complex Breast Reconstruction: Indications, Outcomes and a Proposed Algorithm. Plast Reconstr Surg Glob Open 2019;7:e2382. [Crossref] [PubMed]
- Ishii N, Ando J, Shimizu Y, et al. A novel technique for large and ptotic breast reconstruction using a latissimus dorsi myocutaneous flap set at the posterior aspect, combined with a silicone implant, following tissue expander surgery. Arch Plast Surg 2018;45:484-9. [Crossref] [PubMed]
- Friedman O, Fliss E, Inbal A, et al. Latissimus Dorsi Flap: A Winning Hand for Breast Reconstruction Salvage. Isr Med Assoc J 2019;21:260-4. [PubMed]
- Luce EA, Adams RL, Chandler RG, et al. Tissue Expander versus Tissue Expander and Latissimus Flap in Morbidly Obese Breast Reconstruction Patients. Plast Reconstr Surg Glob Open 2015;3:e323. [Crossref] [PubMed]
- Mohiuddin W, Chevrollier GS, Greaney PJ Jr, et al. Optimizing Results of Postmastectomy Radiation Therapy Utilizing the Latissimus Dorsi Flap and Tissue Expander Technique: A Single-Center Experience. Eplasty 2017;17:e40. [PubMed]
- Taglialatela Scafati S, Cavaliere A, et al. Combining Autologous and Prosthetic Techniques: The Breast Reconstruction Scale Principle. Plast Reconstr Surg Glob Open 2017;5:e1602. [Crossref] [PubMed]
- de Runz A, Boccara D, Bekara F, et al. Outcome of 122 delayed breast reconstruction following post-mastectomy radiotherapy: The scarless latissimus dorsi flap with tissue expansion technique. Ann Chir Plast Esthet 2017;62:23-30. [Crossref] [PubMed]
- Minabe T, Harii K, Imanishi N. Latissimus dorsi flaps oriented on the lateral intercostal artery perforators: anatomical study and application in autologous breast reconstruction. J Plast Surg Hand Surg 2011;45:58-65. [Crossref] [PubMed]
- Leuzzi S, Stivala A, Shaff JB, et al. Latissimus dorsi breast reconstruction with or without implants: A comparison between outcome and patient satisfaction. J Plast Reconstr Aesthet Surg 2019;72:381-93. [Crossref] [PubMed]
- Kallaway C, Humphreys A, Laurence N, et al. Latissimus dorsi myocutaneous reconstruction: a study of long-term outcomes in a district general hospital. Ann R Coll Surg Engl 2016;98:574-7. [Crossref] [PubMed]
- Thomsen JB. Secondary Breast Reconstruction With a Flap of Skin From the Back. ClinicalTrials.gov Identifier: NCT02169011.
- Spear SL, Boehmler JH, Taylor NS, et al. The Role of the Latissimus Dorsi Flap in Reconstruction of the Irradiated Breast. Plast Reconstr Surg 2007;119:1-9. [Crossref] [PubMed]
- Lee JW, Seo JY, Jung YJ, et al. Volumetric changes of the latissimus dorsi muscle after postoperative chemotherapy in cases of immediate breast reconstruction with an extended latissimus dorsi musculocutaneous flap and implant. Gland Surg 2019;8:501-6. [Crossref] [PubMed]
- Browne JP, Jeevan R, Pusic AL, et al. Measuring the patient perspective on latissimus dorsi donor site outcomes following breast reconstruction. J Plast Reconstr Aesthet Surg 2018;71:336-43. [Crossref] [PubMed]
- Rezaei E, Pouryousef K, Karimi M, et al. Latissimus Dorsi Musculocutaneous Flap Inset Innovation in Breast Reconstruction. World J Plast Surg 2019;8:394-400. [PubMed]
- van Huizum MA, Hage JJ, Rutgers EJ, et al. Immediate breast reconstruction with a myocutaneous latissimus dorsi flap and implant following skin-sparing salvage mastectomy after irradiation as part of breast-conserving therapy. J Plast Reconstr Aesthet Surg 2016;69:1080-6. [Crossref] [PubMed]
- Cattelani L, Spotti A, Pedrazzi G, et al. Latissimus Dorsi Myocutaneous Flap in Immediate Reconstruction after Salvage Mastectomy Post-Lumpectomy and Radiation Therapy. Plast Reconstr Surg Glob Open 2019;7:e2296. [Crossref] [PubMed]
- Figus A, Canu V, Iwuagwu FC, et al. DIEP flap with implant: a further option in optimising breast reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1118-26. [Crossref] [PubMed]
- Trignano E, Fallico N, Dessy LA, et al. Transverse Upper Gracilis Flap with Implant In Postmastectomy Breast Reconstruction: a Case Report. Microsurgery 2014;34:149-52. [Crossref] [PubMed]
- Li S, Luan J. Hybrid Prepectoral Breast Reconstruction: A Surgical Approach that Combines the Benefits of Autologous and Implant-Based Reconstruction. Plast Reconstr Surg 2019;144:508e-9e. [Crossref] [PubMed]
- Ad-El DD. Hybrid Prepectoral Breast Reconstruction: A Surgical Approach That Combines the Benefits of Autologous and Implant-Based Reconstruction. Plast Reconstr Surg 2019;144:318e. [Crossref] [PubMed]
- Levine SM, Patel N, Disa JJ. Outcomes of Delayed Abdominal-Based Autologous Reconstruction Versus Latissimus Dorsi Flap Plus Implant Reconstruction in Previously Irradiated Patients. Ann Plast Surg 2012;69:380-2. [Crossref] [PubMed]
Cite this article as: Yesantharao PS, Nguyen DH. Hybrid breast reconstruction: a systematic review of current trends and future directions. Ann Breast Surg 2022;6:17.