A single centre experience in the use of superparamagnetic iron oxide as an alternative tracer in sentinel node biopsy in early breast cancer
Introduction
Sentinel lymph node biopsy using dual radioisotope and Patent Blue dye remains the gold standard in intraoperative assessment of the axilla in early breast cancer (1).
However, difficulties exist in both the worldwide supply of Technetium99 (2) and its use in hospitals without an ARSAC license. With recent changes in delivery of breast cancer services, secondary to pressures from COVID-19, alternative locations for provision of breast cancer surgery have been necessary.
An alternative to radioisotope has been sought to overcome these difficulties, with promising results in the use of superparamagnetic iron oxide (SPIO) (3). The development of a small volume 2 mL of Magtrace® (with SPIO covered by carboxidextran) provides 56 mg of iron with a diameter of <60 nm. This small size allows for the faster migration to the Sentinel node.
Non-inferiority in both false negative rates and ease of use have been reported with Magtrace® and its predecessor Sienna®. Initial results from the Sunrise study (4) of 135 patients as well as both the SentimagIC trial of 148 and Asian retrospective audit of 328 patients (5) suggest the use of magnetic tracer as a safe alternative.
To confirm its use as a viable alternative, we performed a retrospective analysis of its use in all patients between Jan 2019 and Jan 2021 within a large district general hospital. We hypothesize that Magtrace® localisation is as effective as blue dye in the identification of the sentinel node.
We present the following article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-24/rc).
Methods
All patients diagnosed with early breast cancer (T1–3, N0) who underwent a sentinel lymph node biopsy between January 2019 and January 2021 were identified from a unit database.
All patients were assessed with axillary ultrasound preoperatively and discussed as suitable for sentinel node biopsy (SNB) at multidisciplinary team meetings. All procedures were performed by a single surgical team. A review of patient notes including pathology reports and theatre notes was performed to identify all patients where a dual technique of Patent Blue dye and either Sienna® or Magtrace® were used. Pathology reports identified the successful localisation of a sentinel node and its method of identification: whether blue, magnetic tracer positive or both. The SNB was performed in combination with either mastectomy or breast conserving surgery. The intraoperative detection of the sentinel node was performed using Patent Blue dye and either 5 mL of Sienna® or 2 mL of Magtrace®, which were injected subdermally and retroareolar after induction of anaesthesia. Massage was performed for 5 minutes and identification of the sentinel node was performed using Sentimag®. In suitable patients a single incision was performed for both the breast and axillary surgery and the Sentimag® probe was used to assess the axillary bed, after removal of the sentinel node, to ensure all nodes were removed.
Statistical analysis
Data was collated on a Microsoft Excel database and P values were calculated using standard Chi Square and independent t test calculators.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for this study was considered unnecessary by the Ethics Department in our Institution (Southern Health and Social Care Trust) as it was a retrospective study and no patient identifiable data was shared. Informed consent was also waived.
Results
A total of 99 patients were identified in the study; 98 female and 1 male. Eleven patients were administered Sienna® and 88 patients Magtrace® in conjunction with Patent Blue dye. The average age of patients was 59 years with a range from 32–80 years. The average tumour size was 25 mm with a range of 0.3 to 93 mm (Excel Software). Multifocality was present in 19%.
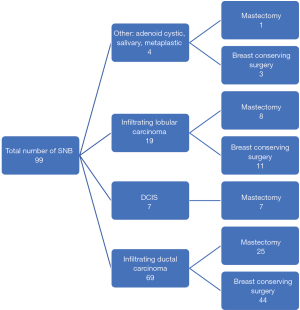
Forty-one patients (41%) had a mastectomy and sentinel node with 3 (3%) having a skin sparing mastectomy and immediate reconstruction. Of those undergoing mastectomy 27 patients (66%) had their surgery after the start of the COVID-19 pandemic and during a timeframe when restrictions were placed on reconstruction. Of the remainder, 5 patients (5%) had a central segmentectomy and 54 patients (54%) had breast conserving surgery with 13 Level 2 therapeutic mammoplasty and 41 level 1 wide local excisions. Of those having breast conserving surgery, 40% had a Magseed® inserted as their method of tumour localisation. Of the 41 mastectomies performed 7 were for ductal carcinoma in situ (DCIS) and 34 for invasive disease. With respect to tumour type, 70% of patients had ductal carcinoma and 19% had lobular carcinoma (see Figure 1).
Patent Blue dye was identified in 94.9% of sentinel nodes and magnetic tracer was identified in 91.9%. In combination, the sentinel node was identified in 97.9% of sentinel lymph node biopsies. There was a failure to identify the sentinel node in 2.1% of patients. Both these patients had a mastectomy. In one case the axilla had a high nodal burden not picked up on ultrasound. An axillary node clearance was performed at the time of SNB and 12 out of 21 nodes were positive with extranodal extension. In the second case there were multifocal tumours and 2 out of 6 nodes were positive in the nodal sample.
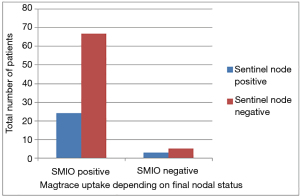
In 3 cases, where the blue dye did not identify the sentinel node, the node was positive for magnetic tracer. In 4 out of a total of 5 cases where blue dye did not travel the SNB was in conjunction with a mastectomy. In only 3 cases the node was negative for magnetic tracer and was involved with cancer spread, giving a false negative rate of 3% (see Figure 2).
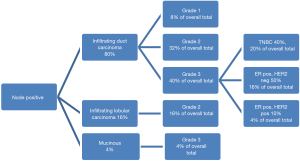
On average, 2.2 nodes were sampled at time of SNB and 25% of all SNBs were node positive (25 patients). Within the node positive cohort the average tumour size was larger at 32 mm with a range of 10.5–72 mm. The median grade was 2 with 48% grade 2 and 44% grade 3. A basal phenotype, i.e., grade 3 infiltrating ductal carcinoma which was triple negative, was associated with 20% of those positive on sentinel node. Given the incidence of triple negative breast cancers as 15% of all breast cancers (6) this would suggest TNBCs as a more aggressive tumour subtype although P=0.71 (see Figure 3).
There were no reported unexpected significant adverse reactions to the combined tracers.
Discussion
The study shows that sentinel lymph node identification using dual modalities is still superior to a single tracer technique with an improvement in sentinel node identification of 3%. The use of dual technique allows a 97.9% identification of sentinel node compared to only 94.9% using blue dye alone. This improvement in localisation highlights the benefit of a dual technique and allows an alternative to radioisotope in a centre where it is not available.
In introducing any new technique, the accuracy and therefore the false negative rate is important. In this series, the false negative rate was 3% for SPIO and 2% for combined dual technique. This is favourable when compared to radioisotope Technetium99 studies where a recent meta-analysis of 9,000 patients reported a false negative rate of 7.4% for radioisotope alone and 5.9% for the dual technique (7).
Of the 11 patients where Sienna was used as a tracer they all demonstrated nodes positive for blue dye and tracer. Given the small sample size of this cohort a P value of significance is not possible.
Analysis of the 25% node positive patients demonstrated a significant difference in tumour size compared with the node negative population (32 mm, 25 mm; P=0.0107 t-test). This would correlate with the already described prognostic value of tumour size in predicting nodal status (8). This is further supported by the fact that both cases where a SLN was not identified were both in mastectomies with a larger tumour size.
We used a Magseed® in 22% of cases and in 40% of breast conserving surgery. We did not experience any difficulty in the use of Magseed® and Magtrace® concurrently but where the Magseed® was placed in the periareolar region a decision was made preoperatively by the multidisciplinary team, to avoid the use of SPIO tracer.
Previous studies have highlighted skin staining with SPIO and have reported rates of 67% with periareolar injection (4). We certainly found cases where staining was noticeable but did not have this as an endpoint during this study. Further use of patient-reported outcome measures should be utilised to quantify the impact this has on patients. Ongoing studies into peritumoral injection of the SPIO are predicted to address this issue (9).
Use of SPIO intra-operatively along with Patent Blue dye reduces need for interventions prior to surgery, avoids difficulties associated with the handling and disposal of radioactive specimens and facilitates the organisation and fluidity of theatre lists with a potential reduction in delays.
Conclusions
We believe that, given the impact of COVID-19 on the delivery of breast cancer surgery, the use of SPIO is a safe and reliable technique in the breast surgeon’s toolkit to facilitate delivery of service in centres not equipped to utilise radioisotopes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-24/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-24/dss
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-24/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-24/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for this study was considered unnecessary by the Ethics Department in our Institution (Southern Health and Social Care Trust) as it was a retrospective study and no patient identifiable data was shared. Informed consent was also waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Early and locally advanced breast cancer: diagnosis and management 2018 [National Institute of Clinical Excellence guideline (NG101)]. Published date: 18 July 2018. Available online: https://www.nice.org.uk/guidance/ng101
- Ruth TJ. The Shortage of Technetium-99m and Possible Solutions. Annu Rev Nucl Sci 2020;70:77-94. [Crossref]
- Alvarado MD, Mittendorf EA, Teshome M, et al. SentimagIC: A Non-inferiority Trial Comparing Superparamagnetic Iron Oxide Versus Technetium99 and Blue Dye in the Detection of Axillary Sentinel Nodes in Patients with Early-Stage Breast Cancer. Ann Surg Oncol 2019;26:3510-6. [Crossref] [PubMed]
- Rubio IT, Rodriguez-Revuelto R, Espinosa-Bravo M, et al. A randomised study comparing different doses of superparmagnetic iron oxide tracer for sentinel node biopsy in breast cancer: The SUNRISE study. Eur J Surg Oncol 2020;46:2195-201. [Crossref] [PubMed]
- Man V, Wong TT, Co M, et al. Sentinel Lymph Node Biopsy in Early Breast Cancer: Magnetic Tracer as the Only Localizing Agent. World J Surg 2019;43:1991-6. [Crossref] [PubMed]
- Swain S. Triple-negative breast cancer: metastatic risk and role of platinum agents 2008 ASCO clinical science Symposium, 2008.
- Mok CW, Tan SM, Zheng Q, Shi L. Network meta-analysis of novel and conventional sentinel lymph node biopsy techniques in breast cancer. BJS Open 2019;3:445-52. [Crossref] [PubMed]
- Sopik V, Narod SA. The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer. Breast Cancer Res Treat 2018;170:647-56. [Crossref] [PubMed]
- Klimberg VS, Rubio IT, Henry R, et al. Subareolar versus peritumoral injection for location of the sentinel lymph node. Ann Surg 1999;229:860-4. [Crossref] [PubMed]
Cite this article as: Scally N, Armstrong L, Mathers H. A single centre experience in the use of superparamagnetic iron oxide as an alternative tracer in sentinel node biopsy in early breast cancer. Ann Breast Surg 2022;6:2.