
Gynecomastia
Introduction
Gynecomastia, which stems from the Greek “gyne” meaning women and “mastos” meaning breast, describes excessive benign development of the male breast(s) due to proliferation of glandular tissue (1,2). Gynecomastia is the most common breast condition in males (3-5) with a prevalence ranging between 30–70% (1,4,6,7) of the population and occurs bilaterally in 50% of patients (3,7). There is lack of consensus on a standard grading scale for gynecomastia with multiple scales, based upon physical exam and fat versus glandular composition, currently being utilized (3,8-11) which are summarized in Table 1.
Table 1
| Grade | Description |
|---|---|
| 1 | Mild hypertrophy ( |
| A | Primarily glandular ( |
| B | Primarily fibrous ( |
| 2 | Moderate hypertrophy ( |
| A | Primarily glandular ( |
| B | Primarily fibrous ( |
| 3 | Severe hypertrophy >500 grams ( |
| 4 | Severe hypertrophy >500 grams ( |
Most gynecomastia is asymptomatic (6). Symptoms may include palpation of a retroareolar breast mass and/or enlargement of the breast(s). Breast pain and tenderness, most prevalent in adolescent gynecomastia, most commonly occurs in the first 6 months of gynecomastia during proliferation of the glandular tissue (7,12). Nipple discharge is very uncommon (13) and should prompt a diagnostic workup as nipple discharge is present in 10% of breast cancers (7). Psychological consequences include depression, anxiety, disordered eating, body dysmorphic disorder, and reduced self- esteem (1,4,14,15).
Traditionally, gynecomastia alone was not thought to have an elevated risk of breast cancer (12,13), however, factors associated with increased incidence of gynecomastia, for instance estrogen exposure and androgen deficiency, may also increase the risk of breast cancer (13,16-18). Brinton et al. performed a meta-analysis indicating a significant association between male breast cancer and gynecomastia (OR 9.78; 95% CI: 7.52–12.71) (19).
Differential diagnoses include diabetic mastopathy (13), benign breast changes, and pseudogynecomastia. Male benign breast disease includes atypical lesions of the breast, dermoid cysts, duct ectasia, fat necrosis, hamartomas, hematomas, intramammary lymph nodes, lymphangiomas, lymphoplasmacytic inflammation, lipomata, mastitis, neurofibroma, sebaceous cysts, and papillomas (12,13). Pseudogynecomastia, also known as lipomastia refers to increased breast size due to fat deposition in the absence of glandular hyperplasia (13). Pseudogynecomastia may be bilateral with or without skin excess and most commonly occurs in obese patients (13).
Etiology
Cases of gynecomastia are usually multifactorial. Hormonal imbalance due to an elevated estrogen to androgen ratio may result in glandular breast proliferation (4,5). The etiology of gynecomastia is typically divided into physiologic and nonphysiologic categories with nonphysiologic gynecomastia further subdivided into pharmacologic, pathologic, and idiopathic causes (5,6,12,13,20). The diagnosis of physiologic and idiopathic gynecomastia, each accounting for approximately 25% of gynecomastia (5,6,12,13), should not be made until other underlying etiologies have been excluded (12).
Physiologic gynecomastia
The term physiologic gynecomastia refers to expected hormonal fluctuations that occur throughout development and aging. The prevalence of physiologic gynecomastia is felt to vary widely based on a trimodal distribution (12) with incidence between 60–90% in neonates (1,3,4), 50–60% in adolescents (1,3,4) and 60–70% in the elderly (also known as gynecomastia of senescence) (1,4,5,12). Neonatal transient breast hypertrophy (13) occurs in up to 90% (12) of newborns due to maternal placental estrogens (5,6,12). The work up of suspicious neonatal gynecomastia should be delayed until at least 1 year of age (4,5). Adolescent physiologic pubertal gynecomastia (7) most commonly occurs between 13 to 15 years old (7,13) and spontaneously regresses in up to 95% (13,21) of cases by 6 months to 2 years (12).
Nonphysiologic gynecomastia
Nonphysiologic gynecomastia encompasses pharmacologic, pathologic and idiopathic etiologies. Nonphysiologic gynecomastia, in contrast to physiologic gynecomastia, can occur at any age (12). The most common cause of nonphysiologic gynecomastia is persistent pubertal gynecomastia and should prompt further workup if persistent beyond 2 years (12).
Another common cause is pharmacologic and the medications that cause this are numerous. These can include antiandrogens, antibiotics, antifungals, antihypertensives, antiretrovirals, chemotherapeutics, environmental exposures, hormones, gastrointestinal agents, psychiatric medications, and other agents. Even significant bilateral testicular trauma may lead to decreased testosterone production and resultant gynecomastia (12). A compilation of pharmacologic agents associated with gynecomastia, based on the quality of evidence in the literature, is listed in Table 2 (3,5,7,12,13,18,20,22-25).
Table 2
| Drug class | Agent |
|---|---|
| Antibiotics | Ethionamide, metronidazole, minocycline, anti-tuberculosis (isoniazid) |
| Antifungals | Ketoconazole* |
| Antiretrovirals | Protease inhibitors |
| Cardiovascular agents | Spironolactone*, calcium channel blockers (nifedipine£, verapamil£, amlodipine, diltiazem, felodipine), angiotensin converting enzyme inhibitors (captopril, enalapril, lisinopril), anti-arrhythmics (amiodarone, digitalis), digoxin, furosemide, methyldopa, reserpine |
| Chemotherapeutics | Alkylating agents£, methotrexate, cyclophosphamide, dasatinib, imatinib |
| Environmental exposure | Phenols, phthalates, phytoestrogens [lavender, tea tree oil, ginseng, hops (beer), tribulus terrestris, herbicides, licorice, black cohosh, red clover, dong quai and high dose soy products (>300mg/daily)], lead, meat or milk products of animals treated with estrogens |
| Gastrointestinal agents | Anti-acids [H2- receptor blockers (cimetidine*, ranitidine), proton pump inhibitors (omeprazole£, lansoprazole, rabeprazole)], prokinetics (domperidone, metoclopramide), misoprostol |
| Hormones | estrogens*, antiandrogens (bicalutamide*, flutamide*, cyproterone acetate*, nilutamide), 5α-reductase inhibitors (dutasteride*, finasteride*, epristeride, alfatradiol), human growth hormone (hGH)*, human chorionic gonadotropin (hCG)*, gonadotropin-releasing hormone (GnRH) analogs (goserelin*, leuprorelin*), anabolic steroids£, androgens, clomiphene citrate, corticosteroids, cyproterone, diethylstilbestrol |
| Miscellaneous | HIV medications [efavirenz£, nucleoside reverse transcription inhibitors (NRTI) (stavudine), protease inhibitors (indinavir, saquinavir)], ethanol/alcohol£, opioids (heroin£, methadone£), anti-convulsants (phenytoin, pregabalin, gabapentin), amphetamines, auranofin, benserazide, certirizine, diethylpropion, entecavir, etretinate, marijuana, mirtazapine, loratadine, phenytoin, penicillamine, anti-lipidemics [statins (atorvastatin, pravastatin, and rosuvastatin), fibrates (fenofibrate)], sulindac, theophylline, thiacetazone |
| Psychiatric agents | Risperidone£, first-generation neuroleptics (thioridazine, trifluperazine, prochlorperazine, perphenazine, sulpiride), atypical anti-psychotics (aripiprazole, clozapine, olanzapine, quetiapine, ziprasidone, haloperidol), anti-depressants [selective serotonin reuptake inhibitors/selective norepinephrine reuptake inhibits (SSRI/SNRI) (fluoxetine, paroxetine, venlafaxine, duloxetine)], benzodiazepines (diazepam), phenothiazine, tricyclic antidepressants |
Level of evidence of strength of correlation of medication with gynecology is indicated by * for good and £ for fair (
Pathologic causes of nonphysiologic gynecomastia have a broad differential (6) including chronic liver disease, chronic renal disease, diabetes, heart failure, thyroid disorders, gastrointestinal abnormalities, and neoplasms, among others. Diabetes, for instance, may lead to diabetic mastopathy characterized as a lymphocytic inflammatory infiltration of the mammary ducts in long-standing type 1 diabetes (13). Gynecomastia also occurs in 10–40% (12) of patients with hypothyroidism but may also occur in hyperthyroidism as well (5). Malnutrition is seen in up to 40% of renal failure patients and has been suggested to also contribute to gynecomastia (12). Meanwhile, primary (testicular) or secondary (central) gonadal failure, pseudohermaphroditism, true hermaphroditism, and androgen resistance syndromes are also causes of gynecomastia (6), with gynecomastia occurring in up to 50–70% of patients with Klinefelter syndrome (47 XXY) (5,12,13) who carry a 20–50 times higher risk for breast cancer than men in the general population (4,13,26). Neoplasms account for the etiology of 3% (12) of gynecomastia cases including adrenocortical and testicular neoplasms. Testicular tumors (Leydig, Sertoli, human chorionic gonadotropin (hCG) producing and choriocarcinomas) are rare, with approximately 10% of these patients presenting with gynecomastia alone (12). Other pathologic causes are myriad, including cystic fibrosis, tuberculosis, hemochromatosis, metabolic syndrome, herpes zoster infection, and myotonic dystrophy.
Work up
History
A thorough history and physical is the mainstay for diagnosis of gynecomastia. This should include a detailed timeline of the patient’s signs and symptoms, including the date of onset. A past medical history and family history, including a history of BRCA germline mutations and Klinefelter’s syndrome (4), should be obtained. Medication, recreational drug, and environmental exposures should be reviewed. Any history of testicular trauma or pathology should also be elucidated (12).
Physical examination
A complete physical examination should be performed with attention paid to the breast, lymphatics, thyroid, abdominal and testicular findings. For patients with true gynecomastia, the breast exam will typically reveal a mobile, concentric disc of firm tissue, measuring at least 2 cm (1,2), located directly beneath the nipple-areolar complex (13). These classic exam findings can typically distinguish true gynecomastia from those patients with pseudogynecomastia or breast cancer. Breast cancer is typically distinguished on physical exam by a unilateral, hard, irregular mass that may be located anywhere in the breast which may have associated skin dimpling, fixation, nipple retraction and/or axillary lymphadenopathy (7,13). Any concern for malignancy, including suspicious lymphadenopathy and/or testicular masses, should prompt a diagnostic work up. Physical examination can differentiate pseudogynecomastia, typically found in obese patients, which typically lacks the discrete, focal, retroareolar firm tissue (13) noted in true gynecomastia.
Laboratory testing
Routine laboratory testing, in the absence of suspicious history or physical examination findings, is not recommended (4,7,13). In significant or concerning cases, after elimination of physiologic causes of gynecomastia, a biochemical evaluation may be considered (5). Such an assessment may include liver function tests, serum creatinine, testosterone, estradiol (E2), follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin, thyroid stimulating hormone (TSH), free thyroxine (T4), sex hormone-binding globulin (SHBG) and beta (β-hCG) (5,12,13) to address many of the common causes noted above. Testosterone and LH, when drawn, need to be measured in the morning at their highest levels given normal circadian rhythm fluctuations (12,13). If total testosterone is borderline or low, then a free testosterone can confirm hypogonadism (7). Serum β-hCG, serum dehydroepiandrosterone sulfate, or urinary 17-ketosteroids can be used to evaluate for testicular, adrenal, and other tumors (12) as a potential cause. If serum estradiol or hCG are elevated then testicular ultrasound should be performed to rule out an underlying malignancy (4).
Imaging
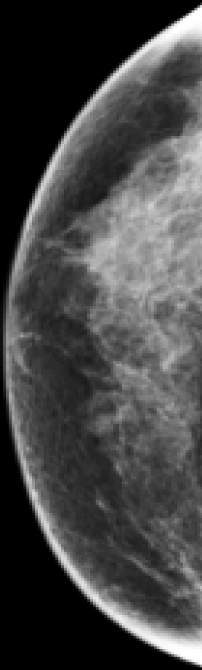
Routine imaging studies are not typically recommended for clear cases of gynecomastia and the need should be guided by physical examination and clinical history (12). Breast imaging, diagnostic mammography, and diagnostic ultrasound, should be performed in those patients having questionable or suspicious physical features. Breast imaging should also be considered in those with a high familial risk, known deleterious BRCA mutation or those with Klinefelter’s syndrome (13), who present with breast concerns. Diagnostic ultrasound is recommended as the initial imaging modality of choice in men less than 25 years of age with an indeterminate palpable mass by The American College of Radiology (18,27). Classic findings of gynecomastia that are pathognomonic include a hypoechoic retroareolar mass that, sometimes poorly defined, is typically flame-shaped as demonstrated in Figure 1 (13). Diffuse glandular enlargement associated with prolonged anti-androgen use is depicted in Figure 2. Clinical suspicion should guide the need for testicular or abdominal imaging to evaluate for testicular or adrenal carcinoma, respectively (12).

Percutaneous biopsy
Percutaneous biopsy is not routinely required if history and exam are consistent with gynecomastia. However, percutaneous biopsy should be considered when carcinoma is unable to be ruled out based upon clinical and imaging findings alone (12,13). Histologic findings of gynecomastia include ductal epithelial hyperplasia with increased stromal and periductal connective tissues (5).
Treatment
The treatment of asymptomatic idiopathic and physiologic gynecomastia, in the absence of features suggesting underlying disease or malignancy, involves sympathetic reassurance and observation (1,12). Ninety percent of cases resolve on their own within a few years (1). Pharmacologic or surgical treatment may be considered in select cases of pubertal gynecomastia for cosmesis, analgesia, or for psychological well-being (12). Biannual follow up may be considered to monitor for resolution (12).
In cases of non-physiologic gynecomastia, treatment of the underlying cause is usually required. Pharmacologically inducted gynecomastia should be treated with discontinuation or substitution of inciting medications, whenever possible, and serial examination for resolution (6). For pathologic gynecomastia, early identification and correction of the suspected acute underlying medical cause can often resolve the gynecomastia in a period as short as one month (4,7). However, longstanding gynecomastia that has been present longer than one year is less likely to regress spontaneously with restoration of hormonal balance because fibrosis is typically present for such cases that will not regress spontaneously (4,7).
Medical treatment
Recommendations for pharmacologic treatment of gynecomastia is limited to studies with small sample sizes, inconsistent methodologies, and lacking control groups (1,3,13). There is no clear consensus on the drug of choice or optimal duration of treatment. Furthermore, the fact that the majority of gynecomastia resolves spontaneously, makes interpretation of these studies challenging (1,7). Longstanding gynecomastia, defined as that greater than 1 to 2 years, is often more refractory to pharmacologic treatment given the underlying hyalinization and fibrosis that occurs over time (3). Tamoxifen, with doses ranging from 10–40 mg, given for 2–4 months has shown improvement in pain scores with regression of gynecomastia in up to 80% of patients (6,7,12,13,28-31). Raloxifene, 60 mg for 3–9 months (32), has also been utilized in the treatment of both pubertal gynecomastia (12,32) and gynecomastia associated with antiandrogen therapy for prostate cancer (12). Anastrozole, given at 1 mg/day (12), has also been used, given its aromatase inhibition, but has not been shown to be more effective than tamoxifen or placebo (6,7,13,33-35) possibly because peripheral aromatization is not the only source of estrogen in the adult male; Leydig cells and germ cells both create estrogen de novo in the testes as well (5), which would not be impacted by aromatase inhibitors. For the treatment of gynecomastia in patients undergoing antiandrogen therapy for prostate cancer, anastrozole appears to be less efficacious than tamoxifen for both prevention and treatment (36,37). Use of dihydrotestosterone (7,38), testolactone (7), danazol (7,12,39-41) and clomiphene citrate (7,12) have been described but have limited supporting literature. Treatment of underlying hypogonadism with testosterone replacement often reduces breast tenderness and gynecomastia (5) in part because testosterone is felt to competitively compete with the estrogen binding to its receptor (42).
Radiotherapy
Radiotherapy has been described for pharmacologic gynecomastia resulting from antiandrogen therapy for prostate cancer (43-47). A randomized controlled trial comparing radiotherapy in a prevention arm to use in a treatment arm, suggests that radiotherapy is most effective if given prophylactically before the administration of antiandrogens (45). However, meta-analysis performed by Viani and colleagues (47) indicate that tamoxifen appears to be two times more effective in the prevention of gynecomastia due to antiandrogen therapy for prostate cancer than radiotherapy.
Surgical therapy
Surgical treatment of gynecomastia is not first-line therapy, and usually only considered in patients with longstanding gynecomastia which is less likely to regress spontaneously or respond to medical treatment given the associated stromal fibrosis (3,4,7). Surgery is also considered in patients who have developed symptoms, including pain or psychologic distress, or aesthetic concerns that have been refractory to initial medical or conservative management attempts. The goal of surgical therapy for gynecomastia is to restore the patient’s ideal body image while minimizing scarring (13). Thorough preoperative counseling should occur to elucidate the patient’s expectations and to help assist the surgeon in determining optimal surgical treatment. For instance, patients who desire chest wall contouring may have more surgical scarring as versus patients that prefer to minimize scarring and opt for a targeted resection (48). Barriers to surgical treatment include high cost and limited insurance coverage (1,49).
Surgical treatment of gynecomastia involves removal of the hypertrophic retroareolar glandular tissue. Attention is given to the contour of the chest, elimination of the inframammary fold, correction of the nipple areolar complex position, removal of redundant skin, and creation of symmetry (13). There are a variety of approaches to the surgical management of gynecomastia which include minimal invasive options, a variety of mastectomy techniques, or a combination of approaches (50). It’s important to remember that this is a contouring procedure and that the goal is not for complete excision of all breast tissue and fat. Removal of all such tissue is traditionally referred to as a subcutaneous mastectomy (7). Meanwhile, excision of the hypertrophic tissue in question may be performed in combination with liposuction to achieve a more cosmetically pleasing appearance (6). Additional considerations include the need to ensure adequate retroareolar tissue, typically a 1 cm even layer of fibrous tissue, in order to prevent contour defects and a saucer deformity (51). Mastectomy techniques can be divided into skin-sparing techniques, mastectomy with a component of skin resection, and simple mastectomy with free nipple grafts based on the severity of the gynecomastia and desired cosmetic outcomes (50). Mild cases of gynecomastia treated surgically can often be approached through a periareolar incision for direct excision of glandular tissue via a crescent or circumareolar incision (Benelli type) (12,50,52). Moderate gynecomastia is often treated with direct excision with associated vertical or Wise pattern mastopexy incision (13), and an inframammary approach (6) for glandular excision with a pedicled nipple-areolar complex or a free nipple graft may be considered in cases of severe gynecomastia.
Minimally invasive approaches have also been described with the use of serial percutaneous biopsy techniques (53), endoscopic and vacuum assisted techniques (3,50,54-56), liposuction (5,50), or use of an ultrasonic scalpel (48). Liposuction is typically most effective for the treatment of adiposity associated with pseudogynecomastia rather than for the treatment of the fibrous glandular hypertrophy of true gynecomastia because of the density of the breast tissue that needs to be removed. However, minimally invasive options may be added to any of the mastectomy approaches outlined above for additional contouring (5,50). The Pull-Through technique involves a combination of minimally invasive incisions with resection of glandular tissue coupled with liposuction (50,52). Improved technology of radiofrequency-assisted liposuction (3) may further assist in removal of both fatty and more glandular tissue however direct excision of glandular tissue is often still required (6,13).
Given the breadth of surgical management options for gynecomastia, there is wide variation in published complications rates, ranging from 0 to 33% with an average of 13.1% (13,50,52). Factors including prolonged symptom duration and severity of gynecomastia have been associated with an increased surgical complication rate (12,48). Hematoma is the most common complication with an average of 5.8% while seroma rates average 2.4% (50). There have also been reports of infection, nipple necrosis and dehiscence (50). Hypoesthesia, which is often transient, ranging in incidence from 3–19% (13,50,52). Revision rates vary from 0–14.1% (50).
Recurrence and long-term outcomes
A study with a mean follow-up of 10.2 months has estimated recurrence rates of gynecomastia between 4.7–12.5% (57) with higher recurrence rates in those patients having lipomatous gynecomastia, defined as isolated adipose tissue hypertrophy, versus those with glandular gynecomastia (21). Long term recurrence rates, mean of 13.8 years, have been shown to be as high as 62.5% in those patients with lipomatous gynecomastia versus 12.5% in those with glandular gynecomastia with no statistically significant difference in BMI between the groups (21). Of note, the study design (21) of Fricke et al. highlights controversy within the current gynecomastia body literature as many studies would have excluded lipomatous gynecomastia as it may be more consistent with pseudogynecomastia. Recurrence may also occur if there is incomplete resection of mammary tissue at the time of surgery.
Adequate planning and alignment of the patient’s expectations is imperative to achieve optimal satisfaction. Exploration of the patient’s goals for surgery is crucial as it may guide treatment choices including use of medications or specific surgical techniques to balance optimal chest wall contouring while minimizing scaring (48). There are limited publications on quality of life data in patients after surgical treatment of their gynecomastia, which is further impacted by the fact that the majority of the existing data is from non-validated questionnaires administered by the patient’s surgeon. Quality of life surveys administered to 47 patients by their plastic surgeons reveal that up to 98% of patients experience a significant improvement in their psychosocial satisfaction (3,58). While there is no validated quality of life questionnaire for post-operative gynecomastia patients, the Breast Evaluation Questionnaire (BEQ), has been altered for use in this patient population and administered to 74 patients by their plastic surgery team. This revealed 62.5% of patients were satisfied to very satisfied with their surgery (59). Davanco et al. utilized the Short-Form 36 (SF-36) in post-operative gynecomastia patients which showed improvement in multiple domains including mental health, general health, functional capacity, social aspects, and vitality (60).
Clinical scenarios
Adolescent gynecomastia
A 14-year of male presents to your clinic with complaints of a tender lump in the left breast which has been present the last several months. He denies any illicit drug use. His past medical and family history is otherwise noncontributory. On physical exam, he has a BMI of 22 kg/m2. He has bilateral well circumscribed fibrous retroareolar masses noted with the left more prominent and more tender than the right. He has no other findings on his clinical breast exam and his complete physical exam is otherwise unremarkable.
Reassurance is provided that his history and clinical exam are consistent with adolescent gynecomastia. No laboratory or imaging workup is needed. Expected course of self-resolution within 2 years is discussed. Emotional support should be provided. A follow up exam in 6 months is recommended. Evidence of underlying psychosocial consequences with may prompt counseling and/or consideration of treatment. If persistent beyond 2 years and/or refractory to medical treatment, consideration can be given to surgical treatment after setting realistic expectations and a thorough discussion of the patient’s goals.
Gynecomastia due to bicalutamide in prostate cancer
A patient presents to your clinic with complaints of bilateral breast enlargement after recently starting bicalutamide. Other than a recent prostate cancer diagnosis, his past medical and family history is unremarkable. His physical exam is notable for a BMI of 34 kg/m2. His breast exam reveals bilateral, symmetric, dense retroareolar masses with no other suspicious masses or findings. His complete physical exam is otherwise unremarkable.
Reassurance should be provided that bicalutamide-induced gynecomastia due to androgen deprivation. No routine laboratory or imaging work up is needed in the absence of suspicious findings. He can be encouraged to follow up with his Urologist to discuss other treatment options for his prostate cancer as cessation of androgen deprivation or change is type of androgen deprivation medication. Watchful waiting is appropriate. However, if the gynecomastia is bothersome to the patient then medical treatment can be considered with Tamoxifen being utilized most commonly. If the patient has a contraindication these approaches or desires alternative treatment options, especially if pain is refractory to other interventions, subcutaneous mastectomy or, in extreme cases, referral to radiation oncology for discussion of therapeutic radiation can be considered.
Conclusions
The current body of literature on gynecomastia lacks consensus on definition, work up and treatment. This lack of standardization leads to significant heterogeneity in the literature and may fail to exclude patients with pseudogynecomastia. Furthermore, current research is limited by small sample size, lack of controls, and research methodologies. A summary of clinical pearls is listed in Table 3. A summary of gynecomastia review articles is listed in Table 4 (1,3,5-7,12-15,20,24,49) while a list of additional articles on medical and surgical treatment of gynecomastia are listed in Table 5 (6,8-10,28-41,47,48,53-56).
Table 3
| Diagnosis is typically made based on clinical history and examination |
| Rule out pseudogynecomastia or breast cancer |
| Routine laboratory and imaging workup is not typically necessary although any suspicion for malignancy requires further evaluation |
| Physiologic gynecomastia is usually treated with reassurance and observation |
| Pathogenic causes of gynecomastia are addressed by treatment of the underlying causes |
| Pharmacologic gynecomastia is treated by discontinuation or conversion of the inciting drug |
| Medications, like tamoxifen, may be used to treat symptomatic or refractory gynecomastia or for gynecomastia associated with antiandrogen therapy for prostate cancer |
| Radiotherapy may be considered for prophylaxis or treatment of gynecomastia associated with antiandrogen therapy for prostate cancer |
| Surgical treatment may be considered in select cases typically involving chest wall contouring with direct excision, or those refractory to other treatments |
Table 4
| Article | Description | Number of studies |
|---|---|---|
| Braunstein, |
Clinical presentation and work up | – |
| Dickson, |
Comprehensive review | – |
| Barros, |
Comprehensive review out of Brasil | – |
| Deepinder, |
Systematic Review | 150 |
| Nuzzi, |
Psychosocial aspects in adolescent GM | – |
| Ladizinski, |
Comprehensive review | – |
| Ordaz, |
Body image and psychological function | – |
| Fagerlund, |
Systematic review | 17 |
| Fagerlund, |
Systematic review in prostate cancer | 11 |
| Rew, |
Psychosocial systematic review | 10 |
| Sansone, |
GM with a focus on hormonal factors | – |
| Baumann, |
Medical and surgical treatment review | – |
| Solli, |
Psychosocial changes after surgery | 6 |
| Holzmer, |
Comprehensive review of surgery | 17 |
GM, gynecomastia.
Table 5
| Article | Description |
|---|---|
| Medical | |
| Buckle, |
Danazol |
| Parker, |
Tamoxifen |
| Eberle, |
DHT-hp in persistent pubertal GM |
| Jones, |
Danazol |
| McDermott, |
Tamoxifen in idiopathic GM |
| Ting, |
Tamoxifen |
| Saltzstein, |
Tamoxifen |
| Lawrence, |
Raloxifene |
| Plourde, |
Anastrozole in pubertal GM |
| Riepe, |
Anastrozole in pubertal GM |
| Perdona, |
Tamoxifen & radiotherapy in bicalutamide induced GM |
| Boccardo, |
Tamoxifen |
| Hanavadi, |
Tamoxifen |
| Mauras, |
Anastrozole in pubertal GM |
| Viani, |
Tamoxifen |
| Surgical | |
| Simon, |
Surgical treatment |
| Colombo-Benkmann, |
Indications for surgery |
| Rohrich, |
Ultrasound-assisted liposuction |
| Prado, |
Arthroscopic-endoscopic cartilage shaver |
| Cordova, |
Algorithm for surgical treatment |
| Benito-Ruiz, |
Minimally invasive surgery |
| He, |
Vacuum-assisted biopsy |
| Li, |
Surgical treatment |
| Cao, |
Endoscopic subcutaneous mastectomy |
| Holzmer, |
Comprehensive review of surgery |
| Medical & surgical | |
| Baumann, |
Review of conservative and surgical management |
DHT-hp, dihydrotestosterone heptanoate; GM, gynecomastia.
Despite these issues, we know that gynecomastia is a prevalent diagnosis which is based on clinical history and examination. Routine laboratory and imaging workup are often unnecessary except for cases where pathologic etiology or breast cancer cannot be ruled out. Treatment is often supportive in nature. However, use of medications and/or surgical intervention may be considered in select patients.
Acknowledgments
Special Thanks to Dr. Catherine Tuite, section chief of breast radiology at Fox Chase Cancer Center, for her image contribution.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Katharine Yao) for the series “A Practical Guide to Management of Benign Breast Disease”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-124). The series “A Practical Guide to Management of Benign Breast Disease” was commissioned by the editorial office without any funding or sponsorship. Both authors have no other conflicts of interest to declare.
Ethical Statement: Both authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ordaz DL, Thompson JK. Gynecomastia and psychological functioning: A review of the literature. Body Image 2015;15:141-8. [Crossref] [PubMed]
- Klang E, Kanana N, Grossman A, et al. Quantitative CT Assessment of Gynecomastia in the General Population and in Dialysis, Cirrhotic, and Obese Patients. Acad Radiol 2018;25:626-35. [Crossref] [PubMed]
- Fagerlund A, Lewin R, Rufolo G, et al. Gynecomastia: A systematic review. J Plast Surg Hand Surg 2015;49:311-8. [Crossref] [PubMed]
- Baumgarten L, Dabaja AA. Diagnosis and Management of Gynecomastia for Urologists. Curr Urol Rep 2018;19:46. [Crossref] [PubMed]
- Sansone A, Romanelli F, Sansone M, et al. Gynecomastia and hormones. Endocrine 2017;55:37-44. [Crossref] [PubMed]
- Baumann K. Gynecomastia - Conservative and Surgical Management. Breast Care (Basel) 2018;13:419-424. [Crossref] [PubMed]
- Braunstein GD. Clinical practice. Gynecomastia. N Engl J Med 2007;357:1229-37. [Crossref] [PubMed]
- Simon BE, Hoffman S, Kahn S. Classification and surgical correction of gynecomastia. Plast Reconstr Surg 1973;51:48-52. [Crossref] [PubMed]
- Rohrich RJ, Ha RY, Kenkel JM, et al. Classification and management of gynecomastia: defining the role of ultrasound-assisted liposuction. Plast Reconstr Surg 2003;111:909-23; discussion 924-25. [Crossref] [PubMed]
- Cordova A, Moschella F. Algorithm for clinical evaluation and surgical treatment of gynaecomastia. J Plast Reconstr Aesthet Surg 2008;61:41-9. [Crossref] [PubMed]
- Ratnam BV. A new classification and treatment protocol for gynecomastia. Aesthet Surg J 2009;29:26-31. [Crossref] [PubMed]
- Gynecomastia Dickson G. Am Fam Physician 2012;85:716-22. [PubMed]
- Barros AC, Sampaio Mde C. Gynecomastia: physiopathology, evaluation and treatment. Sao Paulo Med J 2012;130:187-97. [Crossref] [PubMed]
- Rew L, Young C, Harrison T, et al. A systematic review of literature on psychosocial aspects of gynecomastia in adolescents and young men. J Adolesc 2015;43:206-12. [Crossref] [PubMed]
- Sollie M. Management of gynecomastia-changes in psychological aspects after surgery-a systematic review. Gland Surg 2018;7:S70-6. [Crossref] [PubMed]
- Sasco AJ, Lowenfels AB, Pasker-de Jong P. Review article: epidemiology of male breast cancer. A meta-analysis of published case-control studies and discussion of selected aetiological factors. Int J Cancer 1993;53:538-49. [Crossref] [PubMed]
- Brinton LA, Carreon JD, Gierach GL, et al. Etiologic factors for male breast cancer in the U.S. Veterans Affairs medical care system database. Breast Cancer Res Treat 2010;119:185-92. [Crossref] [PubMed]
- Giordano SH. Breast Cancer in Men. N Engl J Med 2018;378:2311-20. [Crossref] [PubMed]
- Brinton LA, Cook MB, McCormack V, et al. Anthropometric and hormonal risk factors for male breast cancer: male breast cancer pooling project results. J Natl Cancer Inst 2014;106:djt465 [Crossref] [PubMed]
- Ladizinski B, Lee KC, Nutan FN, et al. Gynecomastia: etiologies, clinical presentations, diagnosis, and management. South Med J 2014;107:44-9. [Crossref] [PubMed]
- Fricke A, Lehner GM, Stark GB, et al. Long-Term Follow-up of Recurrence and Patient Satisfaction After Surgical Treatment of Gynecomastia. Aesthetic Plast Surg 2017;41:491-8. [Crossref] [PubMed]
- Braunstein GD. Environmental gynecomastia. Endocr Pract 2008;14:409-11. [Crossref] [PubMed]
- Braunstein GD. Editorial comment: unraveling the cause of HIV-related gynecomastia. AIDS Read 2004;14:38-9. [PubMed]
- Fagerlund A, Cormio L, Palangi L, et al. Gynecomastia in Patients with Prostate Cancer: A Systematic Review. PLoS One 2015;10:e0136094 [Crossref] [PubMed]
- Deepinder F, Braunstein GD. Drug-induced gynecomastia: an evidence-based review. Expert Opin Drug Saf 2012;11:779-95. [Crossref] [PubMed]
- Hultborn R, Hanson C, Kopf I, et al. Prevalence of Klinefelter's syndrome in male breast cancer patients. Anticancer Res 1997;17:4293-97. [PubMed]
- Mainiero MB, Lourenco AP, Barke LD, et al. ACR Appropriateness Criteria Evaluation of the Symptomatic Male Breast. J Am Coll Radiol 2015;12:678-82. [Crossref] [PubMed]
- Hanavadi S, Banerjee D, Monypenny IJ, et al. The role of tamoxifen in the management of gynaecomastia. Breast 2006;15:276-80. [Crossref] [PubMed]
- Parker LN, Gray DR, Lai MK, et al. Treatment of gynecomastia with tamoxifen: a double-blind crossover study. Metabolism 1986;35:705-8. [Crossref] [PubMed]
- McDermott MT, Hofeldt FD, Kidd GS. Tamoxifen therapy for painful idiopathic gynecomastia. South Med J 1990;83:1283-5. [Crossref] [PubMed]
- Perdonà S, Autorino R, De Placido S, et al. Efficacy of tamoxifen and radiotherapy for prevention and treatment of gynaecomastia and breast pain caused by bicalutamide in prostate cancer: a randomised controlled trial. Lancet Oncol 2005;6:295-300. [Crossref] [PubMed]
- Lawrence SE, Faught KA, Vethamuthu J, et al. Beneficial effects of raloxifene and tamoxifen in the treatment of pubertal gynecomastia. J Pediatr 2004;145:71-6. [Crossref] [PubMed]
- Plourde PV, Reiter EO, Jou HC, et al. Safety and efficacy of anastrozole for the treatment of pubertal gynecomastia: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2004;89:4428-33. [Crossref] [PubMed]
- Riepe FG, Baus I, Wiest S, et al. Treatment of pubertal gynecomastia with the specific aromatase inhibitor anastrozole. Horm Res 2004;62:113-8. [PubMed]
- Mauras N, Bishop K, Merinbaum D, et al. Pharmacokinetics and pharmacodynamics of anastrozole in pubertal boys with recent-onset gynecomastia. J Clin Endocrinol Metab 2009;94:2975-8. [Crossref] [PubMed]
- Boccardo F, Rubagotti A, Battaglia M, et al. Evaluation of Tamoxifen and Anastrozole in the prevention of gynecomastia and breast pain induced by bicalutaide monotherapy of prostate cancer. J Clin Oncol 2005;23:808-15. [Crossref] [PubMed]
- Saltzstein D, Cantwell A, Sieber P, et al. Prophylactic tamoxifen significantly reduces the incidence of bicalutamide-induced gynecomastia and breast pain. Br J Urol 2002;90:120-1.
- Eberle AJ, Sparrow JT, Keenan BS. Treatment of persistent pubertal gynecomastia with dihydrotestosterone heptanoate. J Pediatr 1986;109:144-9. [Crossref] [PubMed]
- Ting AC, Chow LW, Leung YF. Comparison of tamoxifen with danazol in the management of idiopathic gynecomastia. Am Surg 2000;66:38-40. [PubMed]
- Jones DJ, Holt SD, Surtees P, et al. A comparison of danazol and placebo in the treatment of adult idiopathic gynaecomastia: results of a prospective study in 55 patients. Ann R Coll Surg Engl 1990;72:296-8. [PubMed]
- Buckle R. Danazol therapy in gynaecomastia; recent experience and indications for therapy. Postgrad Med J 1979;55:71-78. [PubMed]
- Rochefort H, Garcia M. Androgen on the estrogen receptor. I - Binding and in vivo nuclear translocation. Steroids 1976;28:549-60. [Crossref] [PubMed]
- Autorino R, Perdona S, D'Armiento M, et al. Gynecomastia in patients with prostate cancer: update on treatment options. Prostate Cancer Prostatic Dis 2006;9:109-14. [Crossref] [PubMed]
- Di Lorenzo G, Autorino R, Perdona S, et al. Management of gynaecomastia in patients with prostate cancer: a systematic review. Lancet Oncol 2005;6:972-9. [Crossref] [PubMed]
- Widmark A, Fossa SD, Lundmo P, et al. Does prophylactic breast irradiation prevent antiandrogen-induced gynecomastia? Evaluation of 253 patients in the randomized Scandinavian trial SPCG-7/SFUO-3. Urology 2003;61:145-51. [Crossref] [PubMed]
- Eng TY, Abugideiri M, Chen TW, et al. Radiation Therapy for Benign Disease: Arteriovenous Malformations, Desmoid Tumor, Dupuytren Contracture, Graves Ophthalmopathy, Gynecomastia, Heterotopic Ossification, Histiocytosis. Hematol Oncol Clin North Am 2020;34:205-27. [Crossref] [PubMed]
- Viani GA, Bernardes da Silva LG, Stefano EJ. Prevention of gynecomastia and breast pain caused by androgen deprivation therapy in prostate cancer: tamoxifen or radiotherapy? Int J Radiat Oncol Biol Phys 2012;83:e519-24. [Crossref] [PubMed]
- Colombo-Benkmann M, Buse B, Stern J, et al. Indications for and results of surgical therapy for male gynecomastia. Am J Surg 1999;178:60-3. [Crossref] [PubMed]
- Nuzzi LC, Cerrato FE, Erickson CR, et al. Psychosocial impact of adolescent gynecomastia: a prospective case-control study. Plast Reconstr Surg 2013;131:890-6. [Crossref] [PubMed]
- Li CC, Fu JP, Chang SC, et al. Surgical Treatment of gynecomastia: complications and outcomes. Ann Plast Surg 2012;69:510-5. [Crossref] [PubMed]
- Holzmer SW, Lewis PG, Landau MJ, et al. Surgical management of gynecomastia: a comprehensive review of the literature. Plast Reconstr Surg Glob Open 2020;8:e3161 [Crossref] [PubMed]
- Iwuagwu O, Drew P. Minimal invasive surgery for gynecomastia - A novel approach. Can J Plast Surg 2004;12:145-6. [PubMed]
- He Q, Zheng L, Zhuang D, et al. Surgical treatment of gynecomastia by vacuum-assisted biopsy device. J Laparoendosc Adv Surg Tech A 2011;21:431-4. [Crossref] [PubMed]
- Cao H, Yang ZX, Sun YH, et al. Endoscopic subcutaneous mastectomy: A novel and effective treatment for gynecomastia. Exp Ther Med 2013;5:1683-6. [Crossref] [PubMed]
- Prado AC, Castillo PF. Minimal surgical access to treat gynecomastia with the use of a power-assisted arthroscopic-endoscopic cartilage shaver. Plast Reconstr Surg 2005;115:939-42. [Crossref] [PubMed]
- Benito-Ruiz J, Raigosa M, Manzano M, et al. Assessment of a suction-assisted cartilage shaver plus liposuction for the treatment of gynecomastia. Aesthet Surg J 2009;29:302-9. [Crossref] [PubMed]
- Fricke A, Lehner GM, Stark GB, et al. Gynecomastia: histological appearance in different age groups. J Plast Surg Hand Surg 2018;52:166-71. [Crossref] [PubMed]
- Kasielska A, Antoszewski B. Effect of operative treatment on psychosocial problems of men with gynaecomastia. Pol Przegl Chir 2011;83:614-21. [Crossref] [PubMed]
- Ridha H, Colville RJ, Vesely MJ. How happy are patients with their gynaecomastia reduction surgery? J Plast Reconstr Aesthet Surg 2009;62:1473-8. [Crossref] [PubMed]
- Davanço RA, Sabino Neto M, Garcia EB, et al. Quality of life in the surgical treatment of gynecomastia. Aesthetic Plast Surg 2009;33:514-7. [Crossref] [PubMed]
Cite this article as: Sharp NE, Bleicher RJ. Gynecomastia. Ann Breast Surg 2021;5:23.

