
Indocyanine green angiography in breast reconstruction: a narrative review
Introduction
An increasing number of women seek a breast reconstruction, due to increased survival rate after breast cancer (1). A breast reconstruction aims to increase the quality of life and obtain a new breast with an acceptable size, shape and symmetry (2-5). Sufficient perfusion is important in achieving a successful implant-based, oncoplastic- or autologous breast reconstruction. Indocyanine green angiography (ICG-A) is an intraoperative imaging modality visualizing blood flow to the tissue of interest (6-8). The real-time assessment of perfusion supports the surgeon in intraoperative decision making, which consequently leads to a decreased risk of postoperative complications and loss of reconstruction (9-16). We present the following article in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-25/rc).
ICG-A—background
ICG-A has been used to assess skin perfusion for the last two decades (17-19) and is a widely used and well described imaging technique for evaluating tissue perfusion (6,8,20). The modality is not only used to asses arterial perfusion, but has also been described for evaluation of microvascular anastomoses (21,22), venous congestion (23,24), augmentation mastopexy (25), breast reduction surgery (26) and investigation of perfusion zones (27-31).
Scoring and cut-off values in terms of sensitivity, specificity, positive predictive- and negative predictive values have been investigated by several authors (10,11,32-37). In mastectomy flaps, ICG-A has been reported with a sensitivity of 90% and specificity of 100% in reducing skin flap necrosis and overall complication rate (10,38-40). Moyer et al. suggested a cut-off perfusion score of 33% in preventing mastectomy flap necrosis (33). In autologous breast reconstruction establishment of a specific cut-off value and perfusion assessment have yet to be determined (15,41-45).
The majority of published studies on ICG-A in breast reconstruction are of lower level of evidence and consists of comparative, case and cohort studies. Only one randomized controlled trial (RCT)-study investigating ICG-A is published (15). The study investigated the use of ICG-A in deep inferior epigastric artery perforator (DIEP)-flaps and found a significant decreased incidence of fat necrosis (15).
A systematic review from 2020 on the use of ICG-A in autologous breast reconstruction, concluded that per-operative perfusion assessment by ICG-A was an effective tool in reducing fat necrosis compared with flaps assessed clinically (46). Mastectomy skin flap necrosis and the risk of repeated surgeries were reported significantly decreased in 2 reviews and 1 meta-analysis (36,37,47). A Cochrane review on ICG-A on mastectomy skin flap perfusion in immediate breast reconstructions was inconclusive due to lack of high-quality evidence (48). Johnson et al. investigated the overall use of ICG-A in breast reconstructions, and reported a reduced postoperative tissue loss when applying ICG-A, but emphasized the need for standardization (35).
In the following we present a narrative review and a description on how ICG-A may be used in implant-based, oncoplastic- and autologous breast reconstruction demonstrated by clinical examples.
ICG-A—methodology
ICG-A offers an objective, repeatable and real-time imaging of the vascularity and perfusion of tissue (7,49). Indocyanine green (ICG) is a water-soluble molecule excreted via the liver to the bile. The technique is repeatable due to a short half-life of 3–5 minutes. Upon intravenous injection of ICG during surgery a fluorescent near-infrared camera detects the molecule and visualizes perfusion within approximately 20 seconds (6). There is up until now no consensus on the intraoperative dose of ICG which is reported from 2 up to 250 mg (13,50,51).
Several imaging-systems exists among others the Fluobeam Clinical System® (Fluoptics, Grenoble, France, www.fluoptics.com), HyperEye Medical Systems® (Mizuho, Tokyo, Japan, www.mizuhomedical.co.jp) and IC-View® Pulsion Medical Systems. One of the most commonly used systems is the Spy-Elite Fluorescence Imaging Systemâ which is able to quantify perfusion and apply relative values of blood flow in the tissue (33,52). Wearable technology in the form of smart glasses have also been described (53).
Preoperative information
Patients undergoing breast reconstruction should be informed of the rationale and use of per-operative ICG-A. Potential side-effects such as nausea, dizziness, discomfort, rash and sweating occur in up to 0.2–0.34%, and is thoroughly discussed with the patient (32,54-56). Patients allergic to iodine should be excluded due to risk of anaphylaxis (51).
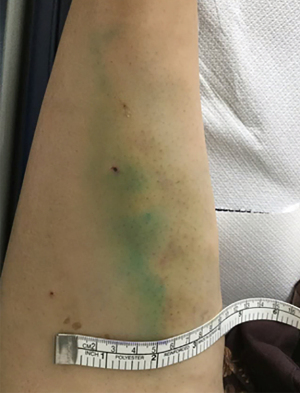
The incidence of anaphylactic shock is rare, and occurs in approximately 1 in 42,000 patients (56). Also, though extravasation is rare, extravasation of ICG may cause reversible discoloration of the skin (Figure 1).
ICG-A—intraoperative application
Implant-based breast reconstruction
Immediate reconstruction
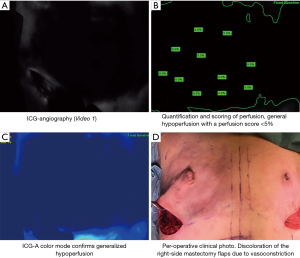
Mastectomy, being it nipple-sparing or skin-sparing, is performed in the plane of the subcutaneous fascia to preserve the dermal blood supply. Hemostasis is secured using bipolar diathermia. After removal of the breast tissue, the surgeon evaluates the skin flaps estimating areas in risk of potential hypoperfusion. The breast surgeon should refrain from using vasoconstrictive agents such as Klein’s fluid (Ringer lactate, lidocaine and adrenaline) to avoid distortion of the assessment of the ICG-A (Figure 2).
A sizer of appropriate size is inserted, and dermis is sutured temporarily. Twenty-five milligrams of ICG are diluted in 10 mL sterile water, an intravenous bolus administration of ICG (Verdyeâ 5 mg/mL) of 2.5 mg/mL is followed by a 10 mL flush with normal saline (2.5 mL of ICG solution for each administration).
The ICG is injected and the perfusion scored by the SPY-Eliteâ system. A perfusion below 33% may lead to reevaluation of the reconstructive procedure by reducing volume of the sizer to eliminate the skin tension (33).
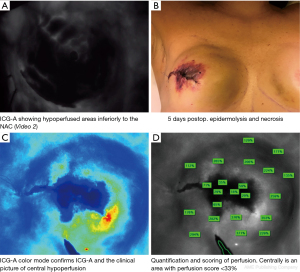
In cases with perfusion below 33% on the first ICG-A, the technique is repeated and re-evaluated using the same dose of ICG, after 20 minutes (6). Consequently, a perfusion <33% on the 2. angiography will result in excision of the hypoperfused area (if located near incision area), a smaller implant or result in reconstruction with subpectoral placement of a tissue expander (TE) (Figure 3).
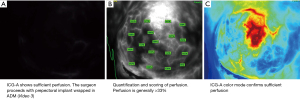
In cases with sufficient perfusion, the reconstruction proceeds with either a pre-pectoral implant wrapped in acellular dermal matrix (ADM) or a subpectoral implant or TE.
After completing the breast reconstruction, ICG-A is then performed again to confirm and ensure sufficient perfusion (Figure 4).
Oncoplastic techniques
Oncoplastic techniques have been used for several decades and can be applied to achieve an acceptable aesthetic result after breast conserving therapy (57-59). Corrective techniques span from Z-plasties and local flaps to larger transposition, advancement and perforator flaps (57). The oncoplastic surgery aims to balance and restore the shape of the breast subsequent to oncologic resection (59). Reshaping and relocation of tissue can compromise perfusion and makes ICG-A a valuable tool in oncoplastic breast surgery (58).
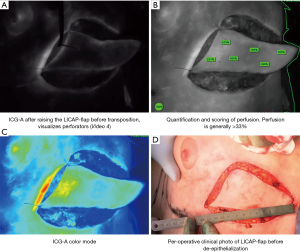
After removing the cancer and intraoperative confirmation of adequate resection, the lateral intercostal artery perforator (LICAP) flap is raised to replace volume and reshape the breast (60). ICG-A can be used per-operatively to assess and score perfusion before after raising the flap, after advancement and before wound closure (Figure 5). In oncoplastic displacement (e.g., breast reduction oncoplasty), the ICG-A technique is used as described for the displacement techniques.
Autologous breast reconstruction
Pedicled flap
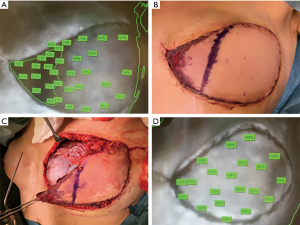
Preoperatively a doppler ultrasonography can be used to mark the perforators or artery(ies) if the chosen pedicled flap is a muscle sparing latissimus dorsi (msLD) or a thoracodorsal artery perforator flap (TAP). Perfusion of the flap can then be scored by ICG-A [as described (33)] performed after incision around the flap to the fascia. The angiography indicates the number of perforators within the flap (Figure 6).
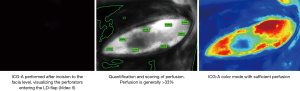
We recommend repeating ICG-A after the flap is completely raised on its pedicle—before transposition/advancement—which allows assessment of the chosen perforator or artery in order to evaluate possible changes in perfusion—assessing the angiosome if the flap is designed as a perforator flap. The final angiography is performed after the flap is transposed to the recipient site. Areas with hypoperfusion (<33%) should be excised.
The angiographies can aid the surgeon in the intraoperative surgical decision making, and the perfusion measurement may identify areas in risk of postoperative necrosis due to hypoperfusion (Figure 7).
Free flap
For breast reconstruction using a free abdominal flap, e.g., deep inferior epigastric artery perforator flap (DIEP), superficial inferior epigastric artery (SIEA) or muscle sparring transverse rectus abdominis (msTRAM) flap, ICG-A can be used to evaluate perfusion of the flap, aiding flap design, identification of perforators and assessing perfusion zones, microvascular anastomoses, venous insufficiency etc.
A preoperative computed tomography angiography (CT-A) is done to identify the perforators and the intramuscular course of the vessels in the flap. By performing ICG-A (as described above) upon incision around the flap to the fascial level—before entering the subfascial plane—the complete number of perforators entering the flap can be identified and compared with the preoperative CT-A.
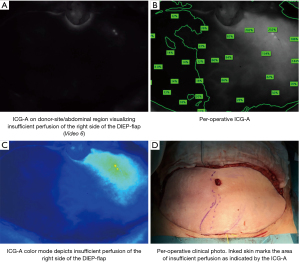
Based on this assessment, the best/most reliable perforators may be dissected, and the angiography repeated, allowing real-time assessment of the perfusion while aiding the intraoperative flap design. If the angiography indicates areas of insufficient perfusion, the surgeon is able to reevaluate and adjust the reconstructive procedure (Figure 8).
After the flap is raised with complete pedicle dissection, ICG-A is repeated allowing a final assessment of flap perfusion before transposition to the breast.
Upon completing the microvascular anastomoses, a repeated angiography may display possible hypoperfused areas of the flap, venous insufficiency or insufficient intra-flap perfusion (Figure 9).
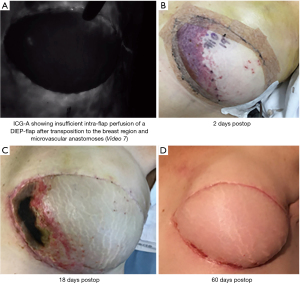
Using ICG-A intraoperatively informs the surgeon of possible insufficiently perfused areas of the flap and aids in reevaluating the breast reconstruction strategy to prevent postoperative complications.
Conclusions
A successful breast reconstruction requires sufficient blood perfusion preventing postoperative complications and loss of reconstruction.
ICG-A provides the surgeon with real-time accurate assessment of the tissue and intraoperative perfusion (7,49). Making information on real-time tissue perfusion available intraoperatively can assist the surgical decision making, providing the opportunity to reevaluate and adapt the reconstruction technique. Repeated intraoperative use of this imaging technique supplies valuable information on perfusion in every step of the reconstruction.
Surgical decision making often relies on clinical experience and judgement. ICG-A can assist the surgeon by providing real-time assessment, scoring and quantification of tissue perfusion.
The role of ICG-A in breast reconstructive procedures is not exhausted.
Determining cut-off values for perfusion, correlating these to postoperative fat necrosis rates or ultimately flap loss remains yet to be investigated. Moreover, further studies, exploring the role of ICG-A in postoperative monitoring, assessment of venous congestion and microvascular anastomoses may further expand the applications of ICG-A in breast reconstructive surgery.
Acknowledgments
We acknowledge Dr. Rami Mossad Ibrahim, MD. Department of Plastic Surgery and Burns Treatment, University Hospital Copenhagen for assisting the photo and video editing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Breast Surgery for the series “Breast Reconstruction - The True Multidisciplinary Approach”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-25/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-25/coif). The series “Breast Reconstruction - The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. TED served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Disclaimer: Videos and clinical photos published with this article are original. Copyright: Dr. Elisabeth Lauritzen, MD, PhD-student, Department of Plastic Surgery and Burns Treatment, University Hospital Copenhagen.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bodilsen A, Christensen S, Christiansen P, et al. Socio-demographic, clinical, and health-related factors associated with breast reconstruction - A nationwide cohort study. Breast 2015;24:560-7. [Crossref] [PubMed]
- Jagsi R, Li Y, Morrow M, et al. Patient-reported quality of life and satisfaction with cosmetic outcomes after breast conservation and mastectomy with and without reconstruction: Results of a survey of breast cancer survivors. Ann Surg 2015;261:1198-206. [Crossref] [PubMed]
- Duggal CS, Metcalfe D, Sackeyfio R, et al. Patient motivations for choosing postmastectomy breast reconstruction. Ann Plast Surg 2013;70:574-80. [Crossref] [PubMed]
- Juhl AA, Christensen S, Zachariae R, et al. Unilateral breast reconstruction after mastectomy - patient satisfaction, aesthetic outcome and quality of life. Acta Oncol 2017;56:225-31. [Crossref] [PubMed]
- Wilkins EG, Hamill JB, Kim HM, et al. Complications in Postmastectomy Breast Reconstruction: One-year Outcomes of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study. Ann Surg 2018;267:164-70. [Crossref] [PubMed]
- Zenn MR. Fluorescent angiography. Clin Plast Surg 2011;38:293-300. [Crossref] [PubMed]
- Griffiths M, Chae MP, Rozen WM. Indocyanine green-based fluorescent angiography in breast reconstruction. Gland Surg 2016;5:133-49. [PubMed]
- Hammer-Hansen N, Juhl AA, Damsgaard TE. Laser-assisted indocyanine green angiography in implant-based immediate breast reconstruction: a retrospective study. J Plast Surg Hand Surg 2018;52:158-62. [Crossref] [PubMed]
- Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 2010;125:1065-73. [Crossref] [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: Results of a prospective trial. Plast Reconstr Surg 2012;129:778e-788e. [Crossref] [PubMed]
- Munabi NCO, Olorunnipa OB, Goltsman D, et al. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: A prospective trial. J Plast Reconstr Aesthet Surg 2014;67:449-55. [Crossref] [PubMed]
- Mattison GL, Lewis PG, Gupta SC, et al. SPY imaging use in postmastectomy breast reconstruction patients: Preventative or overly conservative? Plast Reconstr Surg 2016;138:15e-21e. [Crossref] [PubMed]
- Rinker B. A Comparison of Methods to Assess Mastectomy Flap Viability in Skin-Sparing Mastectomy and Immediate Reconstruction: A Prospective Cohort Study. Plast Reconstr Surg 2016;137:395-401. [Crossref] [PubMed]
- Mirhaidari SJ, Beddell GM, Orlando MV, et al. A Prospective Study of Immediate Breast Reconstruction with Laser-Assisted Indocyanine Green Angiography. Plast Reconstr Surg Glob Open 2018;6:e1774. [Crossref] [PubMed]
- Varela R, Casado-Sanchez C, Zarbakhsh S, et al. Outcomes of DIEP Flap and Fluorescent Angiography: A Randomized Controlled Clinical Trial. Plast Reconstr Surg 2020;145:1-10. [Crossref] [PubMed]
- Chirappapha P, Chansoon T, Bureewong S, et al. Is It Reasonable to Use Indocyanine Green Fluorescence Imaging to Determine the Border of Pedicled TRAM Flap Zone IV? Plast Reconstr Surg Glob Open 2020;8:e3093. [Crossref] [PubMed]
- Eren S, Rubben A, Krein R, et al. Assessment of microcirculation of an axial skin flap using indocyanine green fluorescence angiography. Plast Reconstr Surg 1995;96:1636-49. [Crossref] [PubMed]
- Pestana IA, Coan B, Erdmann D, et al. Early experience with fluorescent angiography in free-tissue transfer reconstruction. Plast Reconstr Surg 2009;123:1239-44. [Crossref] [PubMed]
- Holm C, Mayr M, Höfter E, et al. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg 2002;55:635-44. [Crossref] [PubMed]
- Flower RW, Hochheimer BF. A clinical technique and apparatus for simultaneous angiography of the separate retinal and choroidal circulations. Invest Ophthalmol 1973;12:248-61. [PubMed]
- Mohebali J, Gottlieb LJ, Agarwal JP. Further validation for use of the retrograde limb of the internal mammary vein in deep inferior epigastric perforator flap breast reconstruction using laser-assisted indocyanine green angiography. J Reconstr Microsurg 2010;26:131-5. [Crossref] [PubMed]
- Hitier M, Cracowski JL, Hamou C, et al. Indocyanine green fluorescence angiography for free flap monitoring: A pilot study. J Craniomaxillofac Surg 2016;44:1833-1841. [Crossref] [PubMed]
- Janes LE, Hui-Chou HG, Matthews JA, et al. Utilization of Near-infrared Indocyanine Green Angiography for Immediate and Delayed Venous Outflow Assessment in Breast Reconstruction: A Case Report. Plast Reconstr Surg Glob Open 2014;2:e100. [Crossref] [PubMed]
- Krishnan KG, Schackert G, Steinmeier R. The role of near-infrared angiography in the assessment of post-operative venous congestion in random pattern, pedicled island and free flaps. Br J Plast Surg 2005;58:330-8. [Crossref] [PubMed]
- Swanson E. Safety of vertical augmentation-mastopexy: Prospective evaluation of breast perfusion using laser fluorescence imaging. Aesthet Surg J 2015;35:938-49. [Crossref] [PubMed]
- Murray JD, Jones GE, Elwood ET, et al. Fluorescent intraoperative tissue angiography with indocyanine green: evaluation of nipple-areola vascularity during breast reduction surgery. Plast Reconstr Surg 2010;126:33e-34e. [Crossref] [PubMed]
- Holm C, Mayr M, Höfter E, et al. Interindividual variability of the SIEA angiosome: Effects on operative strategies in breast reconstruction. Plast Reconstr Surg 2008;122:1612-20. [Crossref] [PubMed]
- Holm C, Mayr M, Höfter E, et al. Perfusion zones of the DIEP flap revisited: A clinical study. Plast Reconstr Surg 2006;117:37-43. [Crossref] [PubMed]
- Yamaguchi S, De Lorenzi F, Petit JY, et al. The “perfusion map” of the unipedicled TRAM flap to reduce postoperative partial necrosis. Ann Plast Surg 2004;53:205-9. [Crossref] [PubMed]
- Newman MI, Samson MC. The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J Reconstr Microsurg 2009;25:21-6. [Crossref] [PubMed]
- Anker AM, Prantl L, Strauss C, et al. Clinical Impact of DIEP Flap Perforator Characteristics – A Prospective Indocyanine Green Fluorescence Imaging Study. J Plast Reconstr Aesthet Surg 2020;73:1526-33. [Crossref] [PubMed]
- Gurtner GC, Jones GE, Neligan PC, et al. Intraoperative laser angiography using the SPY system: Review of the literature and recommendations for use. Ann Surg Innov Res 2013;7:1. [Crossref] [PubMed]
- Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: The gray area defined. Plast Reconstr Surg 2012;129:1043-8. [Crossref] [PubMed]
- Koonce SL, Sarik JR, Forleiter CM, et al. A classification system and treatment algorithm for mastectomy flap ischemia in alloplastic breast reconstruction. J Plast Reconstr Aesthet Surg 2020;73:1854-61. [Crossref] [PubMed]
- Johnson AC, Colakoglu S, Chong TW, et al. Indocyanine Green Angiography in Breast Reconstruction: Utility, Limitations, and Search for Standardization. Plast Reconstr Surg Glob Open 2020;8:e2694. [Crossref] [PubMed]
- Liu EH, Zhu SL, Hu J, et al. Intraoperative SPY Reduces Post-mastectomy Skin Flap Complications: A Systematic Review and Meta-Analysis. Plast Reconstr Surg Glob Open 2019;7:e2060. [Crossref] [PubMed]
- Driessen C, Arnardottir TH, Lorenzo AR, et al. How should indocyanine green dye angiography be assessed to best predict mastectomy skin flap necrosis? A systematic review. J Plast Reconstr Aesthet Surg 2020;73:1031-42. [Crossref] [PubMed]
- Harless CA, Jacobson SR. Tailoring through technology: A retrospective review of a single surgeon’s experience with implant-based breast reconstruction before and after implementation of laser-assisted indocyanine green angiography. Breast J 2016;22:274-81. [Crossref] [PubMed]
- Sood M, Glat P. Potential of the SPY intraoperative perfusion assessment system to reduce ischemic complications in immediate postmastectomy breast reconstruction. Ann Surg Innov Res 2013;7:9. [Crossref] [PubMed]
- Diep GK, Hui JYC, Marmor S, et al. Postmastectomy Reconstruction Outcomes After Intraoperative Evaluation with Indocyanine Green Angiography Versus Clinical Assessment. Ann Surg Oncol 2016;23:4080-5. [Crossref] [PubMed]
- Alstrup T, Christensen BO, Damsgaard TE. ICG angiography in immediate and delayed autologous breast reconstructions: peroperative evaluation and postoperative outcomes. J Plast Surg Hand Surg 2018;52:307-11. [Crossref] [PubMed]
- Malagón-López P, Vilà J, Carrasco-López C, et al. Intraoperative Indocyanine Green Angiography for Fat Necrosis Reduction in the Deep Inferior Epigastric Perforator (DIEP) Flap. Aesthet Surg J 2019;39:NP45-54. [Crossref] [PubMed]
- Hembd AS, Yan J, Zhu H, et al. Intraoperative Assessment of DIEP Flap Breast Reconstruction Using Indocyanine Green Angiography: Reduction of Fat Necrosis, Resection Volumes, and Postoperative Surveillance. Plast Reconstr Surg 2020;146:1e-10e. [Crossref] [PubMed]
- Hembd A, Teotia SS, Zhu H, et al. Optimizing perforator selection: A multivariable analysis of predictors for fat necrosis and abdominal morbidity in DIEP flap breast reconstruction. Plast Reconstr Surg 2018;142:583-92. [Crossref] [PubMed]
- Momeni A, Sheckter C. Intraoperative Laser-Assisted Indocyanine Green Imaging Can Reduce the Rate of Fat Necrosis in Microsurgical Breast Reconstruction. Plast Reconstr Surg 2020;145:507e-513e. [Crossref] [PubMed]
- Parmeshwar N, Sultan SM, Kim EA, et al. A Systematic Review of the Utility of Indocyanine Angiography in Autologous Breast Reconstruction. Ann Plast Surg 2020; Epub ahead of print. [Crossref] [PubMed]
- da Silva Neto E, Figueiredo PHM, Moro MG, et al. Use of laser-assisted indocyanine green angiography in breast reconstruction: Systematic review and meta-analysis. J Surg Oncol 2020;121:759-65. [PubMed]
- Pruimboom T, Schols RM, Van Kuijk SM, et al. Indocyanine green angiography for preventing postoperative mastectomy skin flap necrosis in immediate breast reconstruction. Cochrane Database Syst Rev 2020;4:CD013280. [Crossref] [PubMed]
- Burnier P, Niddam J, Bosc R, et al. Indocyanine green applications in plastic surgery: A review of the literature. J Plast Reconstr Aesthet Surg 2017;70:814-27. [Crossref] [PubMed]
- Malagón-López P, Carrasco-López C, García-Senosiain O, et al. When to assess the DIEP flap perfusion by intraoperative indocyanine green angiography in breast reconstruction? Breast 2019;47:102-8. [Crossref] [PubMed]
- Schaafsma BE, Mieog JSD, Hutteman M, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol 2011;104:323-32. [Crossref] [PubMed]
- Available online: https://www.stryker.com/us/en/endoscopy/products/spy-elite.html
- Karakawa R, Yano T, Shibata T, et al. Use of the wearable smart glasses for indocyanine green (ICG) angiography of a flap surgery. Microsurgery 2020;40:276-7. [Crossref] [PubMed]
- Obana A, Miki T, Hayashi K, et al. Survey of complications of indocyanine green angiography in Japan. Am J Ophthalmol 1994;118:749-53. [Crossref] [PubMed]
- Hope-Ross M, Yannuzzi L, Gragoudas E, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:529-33. [Crossref] [PubMed]
- Benya R, Quintana J, Brundage B. Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn 1989;17:231-3. [Crossref] [PubMed]
- Berrino P, Campora E, Santi P. Postquadrantectomy Breast Deformities Classification and Techniques of Surgical Correction. Plast Reconstr Surg 1987;79:567-72. [Crossref] [PubMed]
- Kijima Y, Yoshinaka H, Hirata M, et al. Oncoplastic surgery combining partial mastectomy and immediate volume replacement using a thoracodorsal adipofascial cutaneous flap with a crescent-shaped dermis. Surg Today 2014;44:2098-105. [Crossref] [PubMed]
- Chatterjee A, Gass J, Patel K, et al. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann Surg Oncol 2019;26:3436-44. [Crossref] [PubMed]
- Hakakian CS, Lockhart RA, Kulber DA, et al. Lateral Intercostal Artery Perforator Flap in Breast Reconstruction: A Simplified Pedicle Permits an Expanded Role. Ann Plast Surg 2016;76:S184-90. [Crossref] [PubMed]
Cite this article as: Lauritzen E, Bredgaard R, Bonde C, Jensen LT, Damsgaard TE. Indocyanine green angiography in breast reconstruction: a narrative review. Ann Breast Surg 2022;6:6.

