Tamoxifen use in microvascular breast reconstruction & its effect on microvascular complications: a systematic review & meta-analysis
Introduction
One in eight females will develop breast cancer in their lifetime (1). In the region of forty per cent of women who have a mastectomy undergo reconstructive breast surgery (2). It is becoming an integral aspect of breast cancer treatment and the rate of immediate breast reconstruction is increasing yearly. For women who undergo total mastectomy, breast reconstruction can lead to psychosocial benefits (3).
Autologous reconstruction is considered the gold standard reconstruction. It has the advantage of creating a breast that has a natural feel and has less long-term complications in comparison to implant reconstruction. It does, however, require microvascular anastomosis, which demands additional surgical skill. It is also a major operation to undertake, with an increased risk of systemic complications such as deep vein thrombosis (DVT) and pulmonary embolism. In addition, there is a real risk of a failed reconstruction due to microvascular complications. A failed reconstruction can have significant psychological implications for the patient, including dissatisfaction with their physical appearance (4). The patient may feel like they have lost their breast twice (5). Therefore every effort should be made to minimise the risk of failed breast reconstruction.
Tamoxifen is an adjuvant treatment for those with hormone receptor positive breast cancer. It has been shown to reduce the risk of breast cancer associated mortality, the risk of breast cancer recurrence and the risk of developing a contralateral breast cancer (6,7).
It is estimated that patients with breast cancer alone are at a tenfold higher risk of developing a venous thromboembolism compared to the general population (8). Tamoxifen is also associated with systemic thrombosis, carrying a two to three-fold increased risk in comparison to the general population (9).
As microvascular breast reconstruction and tamoxifen are both independent risk factors for thromboembolic events, it is possible that continuing tamoxifen therapy at the time of surgery may compound this risk. In addition, due to the pro-thrombotic effect tamoxifen has on the systemic circulation, it is possible that it could also impact on the microcirculation at the time of microvascular reconstruction. Therefore, this study aimed to assess if tamoxifen has implications for microvascular thrombosis and secondary thromboembolic events by performing a systematic review and meta-analysis of the literature to guide its use in the perioperative period. We present the following article in accordance with the PRISMA reporting checklist (10) (available at http://dx.doi.org/10.21037/abs-20-57).
Methods
Search strategy
A search of the published literature was performed using the following terms: (“tamoxifen” OR “hormonal therapy” OR “anti-oestrogen” OR “anti-estrogen” OR “selective oestrogen receptor modulator” OR “selective estrogen receptor modulator”) AND (“flap” OR “free tissue”). The following databases were searched, from 1990 until March 2020: Cochrane Library and Central Register of Controlled Trials, PubMed, Science Direct, Embase, Web of Science, Cumulative Index to Nursing and Allied Health (CINAHL) and grey literature databases (including clinicatltrials.gov and http://isrctn.org). Only studies published in the English language were included. The references of all included studies were assessed to identify any other relevant studies. All searches were completed in March 2020. Relevant article titles were then exported to EndNote reference software.
Study identification and selection
Two reviewers (SMB and JFCW) independently screened the titles and abstracts of all studies identified in the search strategy for inclusion. Duplicate articles were deleted. Further assessment of studies identified that potentially met the inclusion criteria then underwent review of the full text article. Articles that were not clearly excluded based on abstract review also underwent full text review. After review of the full text articles, the publications that were deemed relevant were analysed. Articles that reported the outcome on tamoxifen and its impact on microsurgical anastomosis outcomes were included in the study. For inclusion in the systematic review and meta-analysis, the minimum outcome dataset in the publication had to include the number of patients on and off tamoxifen and the incidence of free flap related complications including the number of total flap failures between groups, the number of partial flap failures between groups and the incidence of arterial or venous compromise between those taking tamoxifen and those not on hormonal therapy. Exclusion criteria included the inability to access the publication’s full text. Studies were also excluded if the subjects were animal models or if there was no comparison made between those on tamoxifen and those not on tamoxifen therapy.
Data extraction
Data was collated and then subsequently assessed and analysed according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (11). Two reviewers (SMB and JFCW) independently analysed each study’s methodology, extracted relevant data and input the data into a customised Microsoft excel spreadsheet. Data that was extracted included the name of the first author, the year of publication, the country of publication, patient demographics, the number of patients included, the status of tamoxifen use, flap related and systemic complications. Potential confounding factors including the patient’s smoking status and exposure to pre-reconstruction radiotherapy were also recorded. Primary outcomes of the study were total or partial flap failure and the incidence of arterial or venous microvascular compromise. Secondary outcomes included the incidence of systemic thromboembolic complications.
Statistical analysis
Results were pooled using the software package RevMan 5.3 provided by the Cochrane Collaboration (The Nordic Cochrane Centre, Copenhagen, Denmark) (11). Cases where tamoxifen was continued in the perioperative period were compared with cases with perioperative tamoxifen cessation in patients undergoing microvascular breast reconstruction. The Mantel-Haenszel statistical methodology was employed to assess the primary and secondary endpoints. For dichotomous variables, the treatment effect was calculated using a fixed-effect model and reported as risk ratios (RR) with 95% confidence intervals (CI). This was calculated for each primary and secondary endpoint. Statistical heterogeneity was assessed using the Chi-squared test and the degree of heterogeneity was quantified using the I2 statistic. Significant heterogeneity was assumed if P<0.10 in the Chi-squared test.
Results
Study selection
The search of the literature initially identified 245 articles. These were screened according to title and abstract. Duplicate titles were removed. Further assessment of the article abstracts revealed ten potentially eligible studies. Based on review of the full text article or potentially eligible studies, five studies were deemed eligible to be included in the final meta-analysis (12-16). A flow-chart for the literature screening process and results is seen in Figure 1.
Characteristics of included studies
Details of included studies can be seen in Table 1. Four studies were conducted in USA and one study in France. All studies included were retrospective reviews, with level III evidence. There were no randomised controlled trials identified for inclusion in the study. Sample sizes included in the studies ranged from 78 to 779 patients. The articles assessed patients undergoing treatment between 1993 and 2015.
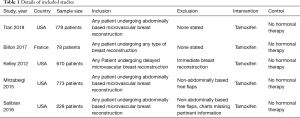
Full table
A total of 2,528 patients were included in the meta-analysis. All five studies compared Tamoxifen use to those not on hormonal therapies and its effect on microvascular complications during free flap breast reconstruction. One study investigated all patients undergoing any type of breast reconstruction. Three studies looked at all patients undergoing abdominally based of microvascular breast reconstruction, whilst the last study looked at patients only undergoing delayed microvascular breast reconstruction.
Outcome analysis
In the five studies evaluated in the analysis, there was a total of 2,528 patients included. Of these, 464 (18.4%) of patients were taking tamoxifen during the perioperative period; 2,065 patients were not on tamoxifen (81.6%) (Table 2). Three studies reported on patient demographics including patient age, body mass index (BMI), and smoking status. All studies reported on the incidence of flaps experiencing partial or total failure. All studies also reported on the incidence of venous and arterial complications. Three studies reported on the incidence of post-operative pulmonary embolism. One study reported on the incidence of post-operative DVT. Three studies reported on the incidence on pre-reconstruction radiotherapy.
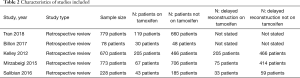
Full table
Patient demographics are detailed in Table 3. The mean age of participants ranged from 45.3 to 55 in patients taking tamoxifen. The mean patient age for those not taking tamoxifen ranged from 49.9 to 52.3 years across the studies. The mean BMI of patients taking tamoxifen ranged from 27.3 to 29.2. The mean BMI of those not taking tamoxifen ranged from 26.8 to 28.9; 11.1% of patients on tamoxifen therapy were smokers; 7.3% of patients not on tamoxifen therapy were active smokers.
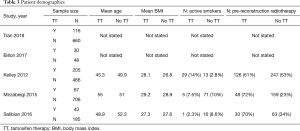
Full table
Primary outcomes
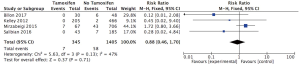
Arterial complications
Four trials reported on arterial complications. There were seven cases of arterial compromise in the tamoxifen subgroup (2%) and fifty-eight cases of arterial compromise in the group not taking tamoxifen in the perioperative period (4.1%). There was no statistically significant difference in the risk of arterial complications between the group taking tamoxifen and the group not taking tamoxifen (RR: 0.88; 95% CI: 0.46 to 1.7; I2=47%) (Figure 2).
Venous complications
The incidence of venous complications was reported by four studies. There were twelve cases of venous compromise in the tamoxifen subgroup (3.5%) and forty-eight cases of venous compromise in the group not taking tamoxifen in the perioperative period (3.4%). There was no statistically significant increased risk associated with tamoxifen use during the perioperative period (RR: 1.11; 95% CI: 0.59 to 2.12; I2=52%) (Figure 3).
Partial flap failure
The incidence of partial flap failure was reported by all five studies. There were ten cases of partial flap failure in the tamoxifen subgroup (2.2%) and forty cases of partial flap failure in the group not taking tamoxifen in the perioperative period (1.9%). Analysis revealed that there was no increased risk associated with tamoxifen use during the perioperative period (RR: 1.06; 95% CI: 0.55 to 2.06; I2=0%) (Figure 4).
Total flap failure
The incidence of total flap failure was reported by all five studies. There was no statistically significant difference in risk with tamoxifen use during the perioperative period (RR: 1.51; 95% CI: 0.81 to 2.83; I2=55%), however, there was a trend toward an increased rate of flap failure in the tamoxifen group; there were thirteen cases of total flap failure in the tamoxifen subgroup (2.8%) and thirty-two cases of total flap failure in the group not taking tamoxifen in the perioperative period (1.55%). There was moderated statistical heterogeneity between the studies (Figure 5).
Secondary outcomes
DVT
Only one study reported the incidence of postoperative DVT among those taking and those not taking tamoxifen in the perioperative period. In the study by Tran et al, one person in the tamoxifen group developed at DVT (0.8%). There were no cases of DVT in the group not taking tamoxifen.
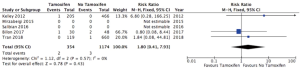
Pulmonary embolism
The incidence of a post-operative pulmonary embolism was recorded by three studies. This included 1,528 patients, of which 354 (23.2%) patients continued tamoxifen therapy during the perioperative period and 1,174 (76.8%) patients were not on tamoxifen. In patients taking tamoxifen during the perioperative period, two patients developed a pulmonary embolism in the post-operative period (0.56%). In patients not taking tamoxifen, three patients developed a post-operative pulmonary embolism (0.26%). There was a trend towards an increased rate of pulmonary embolism in the group on tamoxifen therapy, however, there was no statistically significant increase in risk (RR: 1.8; 95% CI: 0.41 to 7.93, I2=0%) (Figure 6). There was no statistical heterogeneity between these studies.
Discussion
Risk of microvascular thrombosis
It has been theorized over the last decade that because tamoxifen is associated with an increased risk of large vessel thrombosis, it may be associated with an increased risk of microvascular thrombosis and thus may have implications for microvascular breast reconstruction. The effect of tamoxifen on arterial microvascular anastomoses was first investigated in animal models and there was found to be an increased inflammatory infiltrate and intimal thickening in the vessel wall (17). An investigation of the effect of tamoxifen on venous microvascular anastomoses in animal models also saw an increase in wall thickness (18). It has been noted on electron microscopy that in those on tamoxifen, there were activated thrombocytes attached to the vessel wall (18). This meta-analysis showed no statistically significant increased risk in anastomotic arterial or venous microvascular thrombosis. There was, however, a clear trend towards an increased risk in total flap failure in the group of patients who continued tamoxifen in the perioperative period (Figure 5), perhaps indicating intra-flap microvascular thrombotic events. This is in line with two studies, which found a reduced rate of successful flap salvage associated with tamoxifen use (14,16).
Risk of macrovascular thrombosis
During breast reconstruction, a prolonged period of immobility is endured by the patient, with an associated increased incidence of DVT and pulmonary embolism formation. Asymptomatic DVT may be present a third of patients (19). The rate of pulmonary embolism may be as high as 20% (20). The risk of venous thromboembolism may be higher again in bilateral reconstructions (21). As a potentially lethal sequela, every effort should be made to minimise the risk of thrombosis and thromboembolism in the perioperative period.
Tamoxifen is an independent risk factor for venous thromboembolism. In our meta-analysis we found that there was a trend towards an increased risk associated with tamoxifen use and pulmonary embolism, although it was not statistically significant. This is likely due to the low incidence of pulmonary embolism in this meta-analysis, which included 1,528 patients with a pulmonary embolism rate of only 0.33%.
Whilst there is definite evidence to suggest that there is an increased risk of venous thrombosis and thromboembolism, the evidence supporting arterial thrombosis and thromboembolic events such as stroke is less clear. However, one meta-analysis found that there was an increased risk of ischaemic stroke in patients on tamoxifen therapy (22).
When to stop tamoxifen?
There does not seem to be a consensus on whether tamoxifen therapy should be withheld for microvascular breast reconstruction. A survey of plastic surgery units in the UK found that 40% stopped tamoxifen in the perioperative period to reduce the risk of thromboembolism formation (23). The consensus on the duration of cessation varied greatly between the units from one week to six weeks prior to surgery and for one to four weeks after surgery. Tamoxifen has a therapeutic half-life of approximately seven days. Its metabolites, 4-hydroxy tamoxifen and 4-hydroxy N-desmethyl tamoxifen (endoxifen), have half-lives of approximately two weeks (24). Tamoxifen and its metabolites may be retained in tissues and the systemic circulation up to twenty-eight days at levels with sufficient potency. It can even be found in tumour tissue up to several months after cessation of treatment (25). Therefore, tamoxifen should be held at least four weeks preoperatively.
In the UK, guidelines from 2002 recommended the continuation of tamoxifen therapy prior to surgery unless the risk of tamoxifen related thrombosis outweighed the risk of interrupting breast cancer treatment (26). Since then however, there have been a multitude of studies published surrounding the increased risk of systemic thrombosis directly related to tamoxifen therapy. In the product monograph for tamoxifen published by the pharmaceutical company AstraZeneca, they state that its brand, under the trade name of Nolvadex, may increase the risk of complications after microvascular breast reconstruction (27). Microvascular thrombosis is listed in the document as a commonly occurring adverse drug reaction that is seen with tamoxifen, citing the study by Kelley et al. (16). The pharmaceutical company recommends temporarily stopping tamoxifen therapy before delayed microvascular breast reconstruction after assessing the benefits versus the risks of stopping therapy.
There is no clear evidence on when to restart tamoxifen in the postoperative period in the literature or on the product monograph. In addition, there are no studies to our knowledge looking at the implications of withholding tamoxifen for a short duration, or addressing how long it is safe the cease therapy without oncological implications in the breast cancer patient. It has been suggested that tamoxifen be restarted two weeks after surgery for the reason that whilst the majority of microvascular complications occur within the first three days after surgery, they can occur up to twelve days post-op (28). In addition, by this stage patients are generally mobilizing sufficiently to mitigate the risk of deep venous thrombosis or pulmonary embolism. We believe that this is a reasonable time to restart tamoxifen.
Study limitations
This study is a meta-analysis and systematic review based on the published literature looking at the effect that tamoxifen has on microvascular complications during free flap breast reconstruction. Few studies in the literature assessed this endpoint. A limited number of five studies that included a total of only 2,528 patients were identified for inclusion in the meta-analysis. One other relevant study was identified, however, the authors did not provide sufficient detail for inclusion in the meta-analysis. All studies were retrospective reviews. There was heterogeneity between the exact duration that defined active tamoxifen therapy between studies. The sample sizes were small in the studies included. The definition of active tamoxifen therapy differed between studies, with three studies including patients who were actively taking tamoxifen at the time of surgery, and one study including patients who had ceased therapy two weeks prior to surgery, however evidence suggests that tamoxifen may still be systemically active if consumed within four weeks prior to surgery. In addition, there was poor reporting of potential confounding factors for thrombosis such as smoking status and pre-reconstruction radiotherapy between groups and so a conclusion on the impact of covariates was not possible.
Conclusions
In this meta-analysis we found a slightly increased risk of total flap failure associated with tamoxifen use in the perioperative period. We also found a slightly increased risk of pulmonary embolism. Although these were small increased risks, and not statistically significant, there were limited numbers within the included studies, thus alerting to the possibility of a real risk associated with its use. We recommend stopping tamoxifen in the perioperative period to minimise the risk. Based on the current literature and the pharmacokinetics of tamoxifen, we recommend stopping tamoxifen therapy four weeks preoperatively. As the majority of microvascular complications occur within two weeks after reconstruction, we recommend withholding tamoxifen during this time and restarting it two weeks postoperatively.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-57
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-57). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002. CA Cancer J Clin 2002;52:23-47. [Crossref] [PubMed]
- Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: Increasing implant rates. Plast Reconstr Surg 2013;131:15-23. [Crossref] [PubMed]
- Heneghan HM, Prichard RS, Lyons R, et al. Quality of life after immediate breast reconstruction and skin-sparing mastectomy - A comparison with patients undergoing breast conserving surgery. Eur J Surg Oncol 2011;37:937-43. [Crossref] [PubMed]
- Visser NJ, Damen TH, Timman R, et al. Surgical results, aesthetic outcome, and patient satisfaction after microsurgical autologous breast reconstruction following failed implant reconstruction. Plast Reconstr Surg 2010;126:26-36. [Crossref] [PubMed]
- Hamdi M, Andrades P, Thiessen F, et al. Is a second free flap still an option in a failed free flap breast reconstruction? Plast Reconstr Surg 2010;126:375-84. [Crossref] [PubMed]
- EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [Crossref] [PubMed]
- Riggs BL, Hartmann LC. Selective estrogen-receptor modulators -- mechanisms of action and application to clinical practice. N Engl J Med 2003;348:618-29. [Crossref] [PubMed]
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med 2012;9:e1001275 [Crossref] [PubMed]
- Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 2002;360:817-24. [Crossref] [PubMed]
- PRISMA. PRISMA Checklist. Available online: http://www.prisma-statement.org/statement.htm. Accessed 17 December 2014
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions http://handbook.cochrane.org/. Accessed 17 December 2014.
- Tran BNN, Ruan QZ, Cohen JB, et al. Does hormone therapy use increase perioperative complications in abdominally based microsurgical breast reconstruction? Plast Reconstr Surg 2018;141:805e-813e. [Crossref] [PubMed]
- Billon R, Bosc R, Belkacemi Y, et al. Impact of adjuvant anti-estrogen therapies (tamoxifen and aromatase inhibitors) on perioperative outcomes of breast reconstruction. J Plast Reconstr Aesthet Surg 2017;70:1495-504. [Crossref] [PubMed]
- Salibian AA, Bokarius AV, Gu J, et al. The Effects of Perioperative Tamoxifen Therapy on Microvascular Flap Complications in Transverse Rectus Abdominis Myocutaneous/Deep Inferior Epigastric Perforator Flap Breast Reconstruction. Ann Plast Surg 2016;77:630-4. [Crossref] [PubMed]
- Mirzabeigi MN, Nelson JA, Fischer JP, et al. Tamoxifen (Selective Estrogen-Receptor Modulators) and aromatase inhibitors as potential perioperative thrombotic risk factors in free flap breast reconstruction. Plast Reconstr Surg 2015;135:670e-690e. [Crossref] [PubMed]
- Kelley BP, Valero V, Yi M, et al. Tamoxifen increases the risk of microvascular flap complications in patients undergoing microvascular breast reconstruction. Plast Reconstr Surg 2012;129:305-14. [Crossref] [PubMed]
- De Pinho Pessoa BB, Menezes Cavalcante BB, Maia MP, et al. Effect of tamoxifen on arterial microvascular anastomosis. Microsurgery 2007;27:286-8. [Crossref] [PubMed]
- Ceran C, Aksam E, Aksam B, et al. Tamoxifen-related thrombosis: An experimental study in rat venous microvascular anastomosis model. Ann Plast Surg 2017;78:213-6. [Crossref] [PubMed]
- Konoeda H, Yamaki T, Hamahata A, et al. Incidence of deep vein thrombosis in patients undergoing breast reconstruction with autologous tissue transfer. Phlebology 2017;32:282-8. [Crossref] [PubMed]
- Lee JS, Ho Son B, Choi H, et al. Pulmonary Thromboembolism following Mastectomy with Immediate TRAM in the Patients with Breast Cancer: a Prospective Study. J Breast Cancer 2006;9:354-60. [Crossref]
- McKean AR, Knox J, Harris P, et al. Audit of venous thromboembolism in DIEP free flap breast reconstruction. J Plast Reconstr Aesthet Surg 2017;70:970-2. [Crossref] [PubMed]
- Bushnell CD, Goldstein LB. Risk of ischemic stroke with tamoxifen treatment for breast cancer: a meta-analysis. Neurology 2004;63:1230-3. [Crossref] [PubMed]
- Gilleard O, Askouni E, Tavsanoglu Y, et al. Venous thromboembolism prophylaxis for abdominal free tissue breast reconstruction: A multicenter survey. J Plast Reconstr Aesthet Surg 2014;67:733-5. [Crossref] [PubMed]
- Gjerde J, Gandini S, Guerrieri-Gonzaga A, et al. Tissue distribution of 4-hydroxy-N-desmethyltamoxifen and tamoxifen-N-oxide. Breast Cancer Res Treat 2012;134:693-700. [Crossref] [PubMed]
- Lien EA, Solheim E, Ueland PM. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res 1991;51:4837-44. [PubMed]
- MHRA. Committee of Safety of Medicines. Curr Probl Pharmacovigilance; 2002.
- AstraZeneca. NOLVADEX® Prodect monograph. Canada 2015.
- Parikh RP, Odom EB, Yu L, et al. Complications and thromboembolic events associated with tamoxifen therapy in patients with breast cancer undergoing microvascular breast reconstruction: a systematic review and meta-analysis. Breast Cancer Res Treat 2017;163:1-10. [Crossref] [PubMed]
Cite this article as: Beecher SM, Woods JFC. Tamoxifen use in microvascular breast reconstruction & its effect on microvascular complications: a systematic review & meta-analysis. Ann Breast Surg 2021;5:14.