
Staged breast reconstruction before nipple-sparing mastectomy with reconstruction
Since the introduction of the radical mastectomy, surgical treatment for breast cancer has evolved significantly. With the advent of more sophisticated imaging modalities and the development of neoadjuvant chemotherapies (1), surgical technique has progressed from a purely oncologic focus to combining cancer resection with maintaining or restoring cosmesis. Breast-conserving therapy (BCT), which involves lumpectomy with or without axillary dissection, adjuvant radiation therapy, and potentially chemotherapy, has been found to yield the same long-term survival as radical mastectomy (2). Conservative resection, combined with systemic therapies and radiation therapy, has now become the gold-standard for treatment of early-stage (I/II) breast cancers (3). For candidates with larger tumors, especially those in the medial breast who may suffer significant deformity after resection (2), oncoplastic reconstruction may serve as an alternative to mastectomy and whole breast reconstruction. By combining plastic surgery techniques of tissue rearrangement/replacement with resection, larger breast volumes may be removed with satisfactory cosmetic results and equivalent oncologic efficacy (2,4,5). Yet, despite these advancements, there still remains a subset of patients for whom partial resection, no matter the technique, is not an option. Whether for positive margins, large and diffuse disease (6), patient preference, or prophylactic purposes, mastectomy remains an important and necessary procedure for nearly 1/3 of breast cancer patients (7).
Compared to the radical mastectomy, the skin-sparing mastectomy (SSM) is a significant improvement in terms of cosmesis. By preserving the skin envelope, more options for reconstruction are possible. SSM may be followed by reconstruction of the nipple-areolar complex (NAC) as an additional step important for improving patient psychosocial and sexual well-being (8). However, NAC recon has its shortcomings, with the primary concerns being loss of nipple projection over time (9,10) and loss of sensation (11). Recreating the areolar complex also poses difficulties due to the distinct texture and color of the areolar skin. While tattooing is a useful adjunct to recreating the NAC, a significant proportion of patients may need repeat tattooing over the course of months or years to sufficiently achieve or maintain color (11,12). The nipple-sparing mastectomy (NSM) is a further improvement on the SSM; by preserving the NAC, some sensation and the native nipple can be maintained (13). The NSM provides for an improved post-mastectomy quality of life and is a significant predictor of both psychosocial and sexual well-being (14). In comparison to SSM with nipple reconstruction, patients who undergo NSM have higher body image satisfaction, decreased feelings of mutilation, and reduced distress (13).
The NSM has risen in popularity due to its reconstructive advantages. At one institution, after the introduction of the NSM, the number of skin-sparing mastectomies and modified radical mastectomies with reconstruction each decreased to less than 10% of the cases completed, as compared to 65.4% and 34.6% previously (15). Its use in cancer patients was initially considered controversial, however, due to questions regarding oncologic efficacy. Concerns for tumor recurrence within the nipple raised questions for its use (7), but multiple studies have since found that long-term survival and rate of recurrence is comparable in NSM versus the SSM (7,16,17). From an oncologic standpoint, preservation of the NAC can be safely done if it is separated completely from the surrounding glandular and ductal tissue (18), a process that Rusby et al. demonstrated can be performed with a high rate of success ex-vivo (19). The procedure has been found to maintain oncological efficacy even in tumors close to the nipple, with no significant difference in recurrence or disease-free survival found between tumors less than or greater than 2 cm from the nipple (20). However, cases with evidence of NAC or skin involvement by tumor, or cases of inflammatory cancer (21) are absolute contraindications to the procedure.
Preservation of the vascular supply to the NAC is of utmost importance in the NSM, and nipple necrosis is a well-described complication of the procedure, cited to occur in up to 20% of cases (22). While patient selection criteria for NSM eligibility vary, patients with minimal ptosis, A–B cup breasts, BMI <30 kg, and nonsmokers have been generally regarded as ideal candidates for the surgery. Tumor criteria for NSM include size less than 5 cm, tumor location placed far (>2 cm) from the areola, hormone status estrogen receptor (ER)/progesterone receptor (PR)+ and HER2−, and without multifocal disease or lymphovascular invasion (7). On the other hand, high BMI, active smoking, larger breast size (greater than C cup), significant ptosis (grade II/III) and radiation exposure, while not absolute contraindications to the surgery, have been associated with higher complication rates, particularly nipple necrosis (21-23) and nipple malposition (24). Therefore, these characteristics serve as relative contraindications to NSM. A single-institution retrospective review of 675 women undergoing surgery for breast cancer additionally found advanced age and advanced stage of disease were significantly associated with mastectomy without reconstruction. Patients with triple-negative as well as HER2+ genotypes were also less likely to undergo reconstruction, hypothesized to be due to higher risk of recurrence (15) However, some of the traditional eligibility criteria are now being challenged; Schneider et al. reports that patients with large, ptotic breasts may also be candidates for NSM with favorable results (25). NSM has also been offered to patients with larger tumors and short tumor-to-nipple distance with comparable oncologic outcomes (26,27).
Indications and techniques for the NSM may also influence surgical outcomes. Frey et al. found significant differences in patient demographics when separated by indication. Patients who underwent prophylactic NSM were generally of younger age. Therapeutic cases were performed in a population with significantly more radiation and chemotherapy exposure. Prophylactic procedures were also associated with a greater number of inframammary fold (IMF) and vertical incisions, whereas therapeutic cases had a higher proportion of lateral radial incisions and tissue-expander reconstructions (27). Their comparison of 1,212 nipple-sparing mastectomies found equivalent rates of recurrence but a significant increase in infection, implant loss, reconstructive failure, and seroma for therapeutic NSM (27).
Various approaches using surgical delay have been implemented to improve outcomes and broaden indications for NSM. For mastectomies being performed for therapeutic purposes, strategies vary in terms of timing and sequencing of tumor removal and reconstruction.
Oncoplastic reconstruction first with second-stage NSM and reconstruction
For large breasts or those with grade II/III ptosis, NSM may still be an option if up-front oncoplastic reconstruction (volume displacement techniques) is performed first, followed several weeks later by NSM with reconstruction. Economides et al. details this approach for patients in this category who also meet the Georgetown criteria, in which patients qualify for the surgery if they have unifocal disease in a single quadrant of the breast. Additional criteria include tumor size <3 cm, located at least 2 cm from the nipple, without any evidence of skin involvement or inflammatory disease, and with negative margins confirmed on both frozen and permanent sections. In the initial stage, tumor excision, sentinel lymph node biopsy, and oncoplastic reconstruction is performed. Concurrent contralateral mastopexy or reduction mammaplasty is also performed for symmetry. After a 10–12-week delay, the NSM is completed with immediate flap- or implant-based reconstruction, depending on patient preference. If mastectomy skin flap quality is poor at the time of the NSM, reconstruction is delayed. Staging the reconstruction also allows patients to undergo adjuvant chemotherapy in the interim to decrease the tumor burden (28).
In the initial stage, Wise-pattern incisions are often employed with particular attention paid to maintaining the NAC vasculature by preserving superomedial and lateral portions of the skin surrounding the nipple as allowed by the oncologic resection. This is followed by nipple repositioning as appropriate. After allowing 10–12 weeks for healing and improved vascularity, the NSM is completed through an inframammary incision—provided that nipple involvement is excluded through frozen and permanent sections—and followed with reconstruction (28,29).
In one study, this strategy was employed for 50 breasts, 35.1% of which mastectomies were therapeutic. For the 26 patients included in the study, average BMI was 25.7 and mastectomy mass was nearly 500 g, with mean sternal notch-to-nipple distance 28.2 cm. Immediate reconstruction was performed in all patients either with direct-to-implant, tissue expander, or free tissue transfer, and in total, 10% of breasts developed complications requiring reoperation, of which 4% (2 breasts) developed NAC necrosis.
A similar approach has been reported in which up-front tumor removal and oncoplastic reconstruction was performed for patients with macromastia and high-grade ptosis, followed by NSM and reconstruction with abdominally-based autologous tissue transfer after an average of 15 weeks. Of the 61 patients and 122 reconstructions performed, nipple necrosis was seen in nearly 15%, with NAC malposition occurring in 1.6%. Noteworthy in this study was the finding that, though prophylactic and therapeutic groups had no significant differences in complication rates, all cases of NAC necrosis occurred in the therapeutic group after reconstruction and within 6 weeks of the first operation (24). This suggests that longer delay between operations may allow for improved NAC viability.
NSM first with second-stage delayed reconstruction
Another delay strategy has employed performing the NSM up front and completing the reconstruction weeks to months later. Schwartz et al. implements this strategy in a four-stage approach with implant placement for patients at high risk for complications due to their high degree of ptosis (grade III), diabetes, obesity, and macromastia. In the first stage, NSM is completed through the lateral half of the Wise pattern to optimally preserve vasculature in the skin envelope. After about 1.5 weeks of recovery, the remaining half of the Wise pattern incisions are made in an outpatient setting. Nipple repositioning and skin envelope adjustments are performed in a third procedure at a minimum of 10 days later. In this stage of the procedure, care is taken to preserve the IMF and therefore maintain the vascular supply to the NAC through an inferior pedicle of skin. The definitive reconstruction is completed three months after repositioning of the NAC with sub- or pre-pectoral implants through an IMF incision (30).
This technique allows for development of collateral blood flow to the NAC prior to the reconstruction, which is particularly important for high-risk patients (defined as having grade III ptosis, BMI >35, mastectomy weights >1,000 g, or diabetics) who are likely to suffer reconstructive failure. The key lies in staging the Wise pattern incisions and separating the implant placement from steps in adjusting the skin envelope. This staged approach has been shown to lead to successful reconstruction in all of the ten patients in one small series for whom this sequence was performed. Partial nipple necrosis did occur in 2/10 of patients, but this resolved with wound care and reoperation was not necessary (30).
Zenn et al. details a similar approach by completing implant-based reconstruction 2 weeks after NSM. Patients are selected for this procedure if they have C or D cup breasts, with stage I or II ptosis, or have a history of radiation therapy, smoking, or previous surgery. The initial mastectomy is completed ideally through IMF or vertical incision, or radial-lateral if sentinel lymph nodes are involved. After two weeks, the implant-based reconstruction is completed subpectorally using acellular dermal matrix for additional implant support. If the patient desires a larger sized reconstruction than their natural breast, a tissue expander was placed at this stage instead (31). This approach was used for 20 patients, each with at least six months of postoperative follow-up. Of these patients, 75% had implant placement and the remainder received tissue expanders. Two patients experienced superficial necrosis but no nipple necrosis was noted (31).
NSM first with immediate reconstruction with secondary mastopexy/reduction
Up-front NSM with immediate reconstruction can also be a viable option to correct nipple positioning and reshape the breast. Salibian et al. outlines a technique in which NSM with prepectoral implant reconstruction is performed through an IMF incision. Secondary mastopexy is performed on average 24 months after the initial procedure for reduction, reshaping, and symmetry. The secondary incisions are made using a Wise keyhole pattern with a 4-cm diameter around the areola. To provide additional perfusion to the areola, a superior and inferior dermal fat flap is created by creating a 4–6-cm wide strip of deeper de-epithelialization down to the capsule of the implant, extending inferiorly from the nipple to the IMF and superiorly to the new areolar border. If the mastopexy is being performed for reshaping purposes, the medial and lateral flaps are then mobilized, de-epithelialized, and closed. Should a new implant need to be placed, the exchange is performed through a lateral incision in the inferior pedicle with wide undermining of the medial and lateral flaps prior to de-epithelialization. In the secondary procedure, the IMF may be raised for symmetry by tacking the skin to the rib periosteum with sutures placed medially and laterally to the inferior pedicle so as to preserve circulation to the NAC (32).
A similar technique has been employed in autologous reconstructions with success in which initial NSM and immediate autologous reconstruction was followed later by secondary mastopexy. In a group of seventy patients in whom this staging was employed, all of the complications which arose did so after the initial procedure and required only minor interventions. Six o’clock vertical incisions were commonly used for the secondary procedure, but a small portion were completed with lateral or IMF incisions to reposition the nipple and reshape the breast. No patients suffered NAC necrosis after mastopexy. Additionally, success rate for the flaps was 100%, and patient satisfaction was high. This technique relies solely on sub-areolar vascularization from the underlying autologous tissue; because perfusion from the surrounding skin is not needed, larger skin resections are possible without concern for nipple necrosis. Additionally, this allows for use of a variety of incisions without concern for skin necrosis. This has particular utility for patients with ptotic breasts, as it allows for a greater degree of skin envelope manipulation and reshaping with high margin of success (33).
Mastopexy/reduction first with second-stage NSM and reconstruction
Lastly, if the mastectomy is being performed for therapeutic purposes, the skin envelope can be pre-shaped prior to the NSM with delay between mastopexy/reduction and tumor removal. This technique was first pioneered in 2012 by Spear et al., subsequently paving the way for the various previously-described approaches in later years. Again, utilizing the Georgetown criteria for patient selection and those with high-grade ptosis, this procedure repositions the nipple and reduces the skin envelope prior to resection, allowing for a minimum 1-month delay before proceeding with the second portion of the breast removal and reconstruction.
In the first stage, periareolar incisions are made either circumferentially or with a Wise pattern. Care is taken to preserve the vasculature in the superomedial and lateral portions of the areolar dermis when marking and de-epithelializing the new NAC location. If parenchymal resection is necessary for reshaping or reduction, it is taken in a wedge section from the inferior and central aspect of the breast so as not to disturb the perfusion from above. Should patients require neoadjuvant chemotherapy, this would be completed after the first stage, and the second stage of the surgery completed 4–6 weeks after completion of therapy. If chemotherapy is not needed, the second stage is completed a minimum of 3–4 weeks after the first. The NSM is completed through the previous vertical mastopexy scar or through the IMF and then reconstruction is completed with either autologous tissue or implant/tissue expander. Intraoperatively, samples of retroareolar parenchyma are sent for frozen and permanent section; if these are confirmed to be free of tumor involvement, the nipple is preserved, otherwise it’s removed in a separate procedure at a later date (34).
For the 15 patients (24 breasts) who underwent reconstruction with this delay technique, 20% (four breasts) developed complications requiring operative intervention, a rate comparable to that found in traditional NSM. NAC necrosis developed in three breasts (one bilateral patient) requiring debridement, but 23 were successfully reconstructed with no significant deficits to the NAC. In contrast to traditional BCS, which combines oncoplastic reconstruction with lumpectomy followed by radiation at a later date, this technique substitutes NSM for radiation therapy. Therefore, coordination with the patient’s breast surgeon to ensure that the strategy is in line with oncologic goals is a necessity.
This pre-shaping strategy has particular utility for patients undergoing prophylactic mastectomy as well. Of the 24 reconstructions performed in the 2012 Spear study, 71% were for prophylactic purposes. Mastopexy or reduction prior to NSM, with emphasis placed on preservation of the blood supply from the superior portions of the breast, can expand indications for the procedure to patients with traditionally non-ideal surgical characteristics of increased ptosis and moderately large breast size (34). Figure 1 depicts a patient treated with this pre-shaping strategy by the senior author. A 44-year-old woman with a BRCA1 mutation presented for discussion of breast reconstruction after planned prophylactic mastectomies. She underwent up-front bilateral breast reduction with Wise pattern skin incisions (400 grams on right and 364 grams on left). Four months later, she underwent bilateral prophylactic NSM through IMF incisions with immediate free deep inferior epigastric perforator flap reconstruction (Figure 1).

Salibian et al. details how this option may be offered for patients with severe macromastia. Reduction is performed 3–6 months prior to mastectomy and reconstruction, with a mean reduction weight of 383.4 g. In the initial stage, Wise-pattern incisions are created, the IMF portion through which the second stage was completed. Reconstruction was generally completed with either implant or expander, although a small portion (11.1%) underwent autologous reconstruction. Ultimately, average total resected breast mass neared 1,000 g, and in a 1:2 case-matched analysis of the 9 patients/18 breasts which were operated on in this manner, the staged group differed in that its rate of flap necrosis was nonexistent, compared to 22.2% in the non-staged group. There was however one instance of full-thickness NAC necrosis in the staged group necessitating delayed nipple reconstruction.
Staged NSM and reconstruction may also be performed through the vertical reduction scar (35). In a study of 5 patients who had the procedure done for prophylactic purposes, all of whom were classified as having grade III ptosis and large breasts, the IMF incision was deliberately avoided in the second procedure to better preserve vasculature. The reconstruction was then completed with subpectoral implant or tissue expander, ensuring that at least 4 months had first passed after the initial procedure to protect the NAC. Nipples were removed for one patient for whom pathology confirmed lobular carcinoma in situ (LCIS) and postoperatively there was one instance of superficial skin infection and epidermolysis of the NAC, but no cases of necrosis were recorded (35).
It should be noted that this approach, in contrast to the other techniques cited, does not remove the tumor in the initial surgery. Rather, the tumor and NSM are completed several weeks after the initial incisions are made. Previous database studies have established a link between overall and disease-free survival and time between diagnosis and definitive surgery (36). The risk of delaying tumor removal should therefore be discussed with the oncologic surgery team as well as the patient when considering this option.
Nipple delay techniques
A variety of nipple delay techniques have been trialed to reduce NAC necrosis. Initial approaches have involved undermining the parenchymal vasculature under the NAC several weeks to months prior to the NSM. One technique utilizes anesthetic tumescent to achieve this, injecting until a pocket is created under the skin 20-cm wide. The underlying tissue is then undermined from a 5-mm incision made 18cm from the nipple such that the NAC remains perfused solely from the surrounding dermal vasculature, and then antibiotic-impregnated collagen strips are inserted between the nipple and underlying parenchyma before closure. The NSM is completed three weeks later. This technique allows for intraoperative sampling of the posterior portion of the nipple for pathological examination in both the first and second stages to rule out tumor involvement of the NAC. With this technique, NAC necrosis was observed in 1 of 18 patients due to heat injury from the delay procedure, and no tumor relapse was observed in the 21-month averaged follow-up (37).
A similar approach involves mechanically undermining the NAC in advance of the mastectomy. In an outpatient setting, the area is undermined at the same thickness as the plane of the planned mastectomy at least a week prior to removal of the gland, to allow for better perfusion at the time of the NSM. Subareolar biopsy is performed at the time of the NSM, and if confirmed to be negative for tumor involvement on both frozen and permanent sections, the NAC may be preserved. This technique was performed on seven patients deemed pre-operatively to have high risk of necrosis (due to active smoking, significant ptosis, or preexisting surgical scars), and NSM was successfully completed without necrosis for all subjects (38). Further improvements were made on this technique since this time; at the time of the initial delay procedure, an additional 4–5 cm of surrounding skin can be undermined and subareolar biopsies taken prior to the NSM for permanent pathology. Emphasis is placed on maintaining circumareolar perfusion by making either an inferior vertical incision to the IMF or a lateral incision to the axilla rather than periareolar incision. At the time of the mastectomy, a “hemi-batwing” incision is made on the superior aspect of the breast to remove excess skin for those with ptotic breasts and to elevate the NAC; if this incision is made, the skin inside the hemi-batwing is not undermined during the initial delay procedure. Of the 31 procedures performed with this updated technique, 28 nipples were preserved (3 were removed for positive tumor involvement or patient preference) without necrosis (39). Subsequent groups utilizing this technique have reported similar success (40-43).
Another strategy in delaying the NAC involves creating a physical barrier between the nipple and the underlying breast tissue. In one study, 2–3 weeks prior to the scheduled NSM, skin flaps were raised to undermine the NAC and a silicone sheet was placed in the dissected pocket. This served as a barrier from revascularization from the underlying breast tissue and forced the nipple to rely on the blood supply from the subdermal plexus. In one cohort series utilizing this strategy, average nipple delay time was 17.6 days and no signs of NAC compromise were seen in the nipple delay group (45 breasts) compared to 9 breasts (out of 75 breasts) in the non-delay group who showed signs of NAC compromise (44).
Regardless of strategy of staging, with improvements in both oncological treatment options for patients and surgical techniques, more and more women are being deemed candidates for NSM if a staged approach may be employed. In the senior author’s practice, patients presenting for prophylactic mastectomy and reconstruction who have grade II ptosis or higher are offered up-front mastopexy or mammaplasty reduction, which is later followed by NSM and concurrent reconstruction.
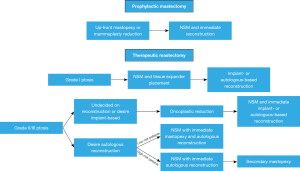
Patients undergoing therapeutic mastectomy with mild ptosis (grade I) undergo NSM with low-height tissue expander placement (often prepectoral) to allow for upper pole contraction and elevation of the NAC. This is followed in a second stage by the reconstructive option of their choice. Those undergoing therapeutic mastectomy with high grade ptosis are offered either oncoplastic reduction or NSM up-front. If women opt for oncoplastic reduction initially, NSM and concurrent reconstruction (either implant or autologous-based) is completed in a second stage. For patients who are undergoing autologous reconstruction who opt for NSM up front, timing of breast envelope shaping depends on whether the patient has a high- or low-risk profile for complications. For high-risk patients (smokers, high BMI, high mastectomy weight, diabetics), patients undergo NSM with autologous reconstruction initially and mastopexy in a second stage. For low-risk patients, NSM, autologous reconstruction and mastopexy may be completed concurrently (Figure 2).
In discussing surgical options with patients, additional factors such as cost and patient psychological well-being should also be factored into the decision-making process. A 2017 study of cost for various therapies for breast cancer found that complications associated with reconstruction cost on average an additional $9,017, as compared to the $512 for complications associated with mastectomy alone (45). However, the overall cost associated with staged reconstruction in particular is not known. Staged reconstruction requires that the patient undergo more procedures over a prolonged period of time as compared to one-stage reconstruction. Although ideally this would reduce incidence and therefore cost of postoperative complications, patients who undergo multi-stage reconstruction may incur increased costs as compared to those who undergo one-stage reconstruction. The additional number of procedures may additionally affect patients’ emotional and psychological wellbeing. Some studies have started to report whether or not patients would be able to tolerate the intermediary state of having deflated breasts, though this factor is not often discussed (31). In choosing a reconstructive approach, the cost as well as the tolerability of multiple procedures should be taken into account.
Several options for staging reconstruction for NSM exist and have low rates of complications if performed appropriately. Ultimately, surgical treatment options should come down to patient preference, risk profile, and a detailed conversation between patient, oncologic surgeon and reconstructive surgeon about timing and ultimate goals.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dung Nguyen) for the series “Cutting-edge of Complex Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-95). The series “Cutting-edge of Complex Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Plesca M, Bordea C, El Houcheimi B, et al. Evolution of radical mastectomy for breast cancer. J Med Life 2016;9:183-6. [PubMed]
- Kaviani A, Sodagari N, Sheikhbahaei S, et al. From radical mastectomy to breast-conserving therapy and oncoplastic breast surgery: a narrative review comparing oncological result, cosmetic outcome, quality of life, and health economy. ISRN Oncol 2013;2013:742462. [Crossref] [PubMed]
- Kaufman CS. Increasing Role of Oncoplastic Surgery for Breast Cancer. Curr Oncol Rep 2019;21:111. [Crossref] [PubMed]
- Santos G, Urban C, Edelweiss MI, et al. Long-Term Comparison of Aesthetical Outcomes After Oncoplastic Surgery and Lumpectomy in Breast Cancer Patients. Ann Surg Oncol 2015;22:2500-8. [Crossref] [PubMed]
- Clough KB, Van la Parra RFD, Thygesen HH, et al. Long-term Results After Oncoplastic Surgery for Breast Cancer: A 10-year Follow-up. Ann Surg 2018;268:165-71. [Crossref] [PubMed]
- Emiroğlu M, Sert İ, İnal A. The Role of Oncoplastic Breast Surgery in Breast Cancer Treatment. J Breast Health 2015;11:1-9. [Crossref] [PubMed]
- Headon HL, Kasem A, Mokbel K. The Oncological Safety of Nipple-Sparing Mastectomy: A Systematic Review of the Literature with a Pooled Analysis of 12,358 Procedures. Arch Plast Surg 2016;43:328-38. [Crossref] [PubMed]
- Bykowski MR, Emelife PI, Emelife NN, et al. Nipple-areola complex reconstruction improves psychosocial and sexual well-being in women treated for breast cancer. J Plast Reconstr Aesthet Surg 2017;70:209-14. [Crossref] [PubMed]
- Momeni A, Ghaly M, Gupta D, et al. Nipple Reconstruction: Risk Factors and Complications after 189 Procedures. Eur J Plast Surg 2013;36:633-8. [Crossref] [PubMed]
- Momeni A, Becker A, Torio-Padron N, Iblher N, et al. Nipple reconstruction: evidence-based trials in the plastic surgical literature. Aesthetic Plast Surg 2008;32:18-20. [Crossref] [PubMed]
- Nimboriboonporn A, Chuthapisith S. Nipple-areola complex reconstruction. Gland Surg 2014;3:35-42. [PubMed]
- Goh SC, Martin NA, Pandya AN, et al. Patient satisfaction following nipple-areolar complex reconstruction and tattooing. J Plast Reconstr Aesthet Surg 2011;64:360-3. [Crossref] [PubMed]
- Didier F, Arnaboldi P, Gandini S, et al. Why do women accept to undergo a nipple sparing mastectomy or to reconstruct the nipple areola complex when nipple sparing mastectomy is not possible. Breast Cancer Res Treat 2012;132:1177-84. [Crossref] [PubMed]
- Wei CH, Scott AM, Price AN, et al. Psychosocial and Sexual Well-Being Following Nipple-Sparing Mastectomy and Reconstruction. Breast J 2016;22:10-7. [Crossref] [PubMed]
- Susini T, Renda I, Giani M, et al. Changing Trends in Mastectomy and Breast Reconstruction. Analysis of a Single-institution Experience Between 2004-2016. Anticancer Res 2019;39:5709-14. [Crossref] [PubMed]
- De La Cruz L, Moody AM, Tappy EE, et al. Overall Survival, Disease-Free Survival, Local Recurrence, and Nipple-Areolar Recurrence in the Setting of Nipple-Sparing Mastectomy: A Meta-Analysis and Systematic Review. Ann Surg Oncol 2015;22:3241-9. [Crossref] [PubMed]
- Galimberti V, Vicini E, Corso G, et al. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast 2017;34:S82-4. [Crossref] [PubMed]
- Niemeyera M, Ettla J, Plattnera B, et al. Nipple-Sparing Mastectomy - Extended Indications and Limitations. Breast Care (Basel) 2010;5:253-8. [PubMed]
- Rusby JE, Kirstein LJ, Brachtel EF, et al. Nipple-sparing mastectomy: lessons from ex vivo procedures. Breast J 2008;14:464-70. [Crossref] [PubMed]
- Balci FL, Kara H, Dulgeroglu O, et al. Oncologic safety of nipple-sparing mastectomy in patients with short tumor-nipple distance. Breast J 2019;25:612-8. [Crossref] [PubMed]
- Tousimis E, Haslinger M. Overview of indications for nipple sparing mastectomy. Gland Surg 2018;7:288-300. [Crossref] [PubMed]
- Rossi C, Mingozzi M, Curcio A, et al. Nipple areola complex sparing mastectomy. Gland Surg 2015;4:528-40. [PubMed]
- Ashikari AY, Kelemen PR, Tastan B, et al. Nipple sparing mastectomy techniques: a literature review and an inframammary technique. Gland Surg 2018;7:273-87. [Crossref] [PubMed]
- Momeni A, Kanchwala S, Sbitany H. Oncoplastic Procedures in Preparation for Nipple-Sparing Mastectomy and Autologous Breast Reconstruction: Controlling the Breast Envelope. Plast Reconstr Surg 2020;145:914-20. [Crossref] [PubMed]
- Schneider LF, Chen CM, Stolier AJ, et al. Nipple-sparing mastectomy and immediate free-flap reconstruction in the large ptotic breast. Ann Plast Surg 2012;69:425-8. [Crossref] [PubMed]
- Dent BL, Miller JA, Eden DJ, et al. Tumor-to-Nipple Distance as a Predictor of Nipple Involvement: Expanding the Inclusion Criteria for Nipple-Sparing Mastectomy. Plast Reconstr Surg 2017;140:1e-8e. [Crossref] [PubMed]
- Frey JD, Salibian AA, Karp NS, et al. Comparing Therapeutic versus Prophylactic Nipple-Sparing Mastectomy: Does Indication Inform Oncologic and Reconstructive Outcomes. Plast Reconstr Surg 2018;142:306-15. [Crossref] [PubMed]
- Economides JM, Graziano F, Tousimis E, et al. Expanded Algorithm and Updated Experience with Breast Reconstruction Using a Staged Nipple-Sparing Mastectomy following Mastopexy or Reduction Mammaplasty in the Large or Ptotic Breast. Plast Reconstr Surg 2019;143:688e-697e. [Crossref] [PubMed]
- Spear SL, Hannan CM, Willey SC, et al. Nipple-sparing mastectomy. Plast Reconstr Surg 2009;123:1665-73. [Crossref] [PubMed]
- Schwartz JC. A New Approach to Nipple-sparing Mastectomy and Reconstruction in the High Risk Ptotic Patient. Plast Reconstr Surg Glob Open 2018;6:e1779. [Crossref] [PubMed]
- Zenn MR. Staged immediate breast reconstruction. Plast Reconstr Surg 2015;135:976-9. [Crossref] [PubMed]
- Salibian AH, Harness JK, Mowlds DS. Secondary Mastopexy After Nipple-Sparing Mastectomy and Staged Subcutaneous Expander/Implant Reconstruction. Ann Plast Surg 2018;80:475-80. [Crossref] [PubMed]
- DellaCroce FJ, Blum CA, Sullivan SK, et al. Nipple-Sparing Mastectomy and Ptosis: Perforator Flap Breast Reconstruction Allows Full Secondary Mastopexy with Complete Nipple Areolar Repositioning. Plast Reconstr Surg 2015;136:1e-9e. [Crossref] [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [Crossref] [PubMed]
- Tondu T, Thiessen F, Tjalma WA. Prophylactic Bilateral Nipple-sparing Mastectomy and a Staged Breast Reconstruction Technique: Preliminary Results. Breast Cancer (Auckl) 2016;10:185-9. [Crossref] [PubMed]
- Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol 2016;2:330-9. [Crossref] [PubMed]
- Palmieri B, Baitchev G, Grappolini S, et al. Delayed nipple-sparing modified subcutaneous mastectomy: rationale and technique. Breast J 2005;11:173-8. [Crossref] [PubMed]
- Jensen JA, Orringer JS, Giuliano AE. Nipple-sparing mastectomy in 99 patients with a mean follow-up of 5 years. Ann Surg Oncol 2011;18:1665-70. [Crossref] [PubMed]
- Jensen JA, Lin JH, Kapoor N, et al. Surgical delay of the nipple-areolar complex: a powerful technique to maximize nipple viability following nipple-sparing mastectomy. Ann Surg Oncol 2012;19:3171-6. [Crossref] [PubMed]
- Martinovic ME, Pellicane JV, Blanchet NP. Surgical Delay of the Nipple-Areolar Complex in High-risk Nipple-sparing Mastectomy Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e760. [Crossref] [PubMed]
- Bertoni DM, Nguyen D, Rochlin D, et al. Protecting Nipple Perfusion by Devascularization and Surgical Delay in Patients at Risk for Ischemic Complications During Nipple-Sparing Mastectomies. Ann Surg Oncol 2016;23:2665-72. [Crossref] [PubMed]
- Dabek RJ, McUmber H, Driscoll D. Surgical Delay in Nipple-sparing Mastectomy. Ann Surg 2018;268:e38-9. [Crossref] [PubMed]
- Zarba Meli E, Cattin F, Curcio A, et al. Surgical delay may extend the indications for nipple-sparing mastectomy: A multicentric study. Eur J Surg Oncol 2019;45:1373-7. [Crossref] [PubMed]
- Martinez CA, Reis SM, Boutros SG. The Nipple-Areola Preserving Mastectomy: The Value of Adding a Delay Procedure. Plast Reconstr Surg Glob Open 2016;4:e1098. [Crossref] [PubMed]
- Smith BD, Jiang J, Shih YC, et al. Cost and Complications of Local Therapies for Early-Stage Breast Cancer. J Natl Cancer Inst 2016;109:djw178. [Crossref] [PubMed]
Cite this article as: Lin A, Shakir A, Garza RM. Staged breast reconstruction before nipple-sparing mastectomy with reconstruction. Ann Breast Surg 2021;5:36.

