
Protecting nipple-areolar complex perfusion by devascularization and surgical delay
Introduction
Nipple sparing mastectomies are rapidly becoming a standard of care option for a vast proportion of women requiring or choosing mastectomy surgery (1-3). Preservation of the nipple areolar complex (NAC) and skin envelope, results in a normal-appearing reconstructed breast. Safeguarding blood flow to the skin is essential to preventing ischemic complications.
A practical approach to circumvent skin loss or necrosis of the NAC, is staging surgical procedures. Briefly, this entails a surgical intervention directed at enhancing blood flow specifically aimed at preserving the integrity of the NAC prior to a delayed mastectomy. Breast surgical oncologists and plastic reconstructive surgeons must collaborate to determine when staged procedures are indicated in order to reduce the risk of ischemia at the time of definitive mastectomy while taking individual anatomic characteristics of a patient into consideration. The timing of these staged procedures is tailored to whether the planned nipple-sparing mastectomy surgery is performed for prophylaxis or cancer treatment.
Our enthusiasm and use of devascularization techniques for the NAC as a first stage operative intervention for a delayed nipple sparing mastectomy has increased over time (4). Risk factors for ischemic complications associated with nipple-sparing mastectomies include vascular perfusion patterns to the NAC, breast size, ptosis, smoking, scars, body mass index (BMI), incision location, and volume or weight of reconstruction (5). Given the inherent risk of ischemic complications with a one stage NSM for many women, alternative procedures such as devascularization of the NAC followed by delayed mastectomy is warranted. Below we discuss and review the role of staged nipple-sparing mastectomies in combination with plastic reconstructive procedures in the context of common clinical scenarios and cancer related treatments.
Ischemic complications after NSM
The most feared complication of a NSM is NAC necrosis. Rates of NAC ischemic complications post NSM vary widely in the literature with more recent studies ranging from 2–64% (3,6-8). Commonly associated risks include ones previously discussed, such as ptosis, smoking, BMI and vascular perfusion patterns. However, additional risks such as cardiovascular disease and older age are noted in a recent study (9). Additionally, ischemic complications can be partial or full. In a recent study by Margenthaler et al. 18.9% of the patients undergoing NSM had overall wound complications while only 3.4% has NAC necrosis requiring resection (10). Similarly, Chirappapha et al. previously reported a 12.1% rate of partial necrosis and 3.5% complete necrosis and associated the risk of these events with greater mastectomy weights (6).
Descriptions of ischemic complications vary widely, ranging from epidermolysis, to partial necrosis or full-thickness necrosis. Epidermolysis usually resolves completely. Healing can occur with skin discoloration. Tissue necrosis manifests itself by partial or complete NAC or skin loss. Most ischemic changes are typically managed by local wound care and limited office-based debridement. However, full thickness necrosis requires surgical debridement and may lead to NAC loss if severe ischemic compromise encompasses a majority of the NAC.
Incision placement has been reported as an additional factor associated with NAC ischemic changes (11). Inframammary fold incisions resulted in lower NAC necrosis in this series of 87 patients undergoing autologous tissue reconstruction but an increased risk for mastectomy skin flap necrosis was noted (12). Radial or radial-lateral incisions are associated with 97–100% nipple survival, in contrast to incisions that extend around the areola or across the NAC (11,13). Similarly, in a study by Ahn et al., periareolar incisions had a 4-fold risk for NAC necrosis compared to other incisions (14). Additionally, superior half periareolar incisions were 2.5 times more likely than a lower half periareolar incisions result in NAC necrosis. In a systematic review, NAC necrosis rate was noted in 7% of 6,615 patients (15). Transareolar incisions compared to radial incisions were associated with a higher rate of NAC ischemic complications, 17.8% versus 9%, respectively.
Another important risk factor associated with NAC ischemia is the type of reconstruction performed, implant based versus autologous tissue. Prosthetic based reconstructions are associated with a lower risk of NAC ischemia and skin necrosis, with the exception of women with lower BMIs (8,11). Endara et al. reported a nearly 4-fold higher rate of NAC necrosis associated with autologous tissue reconstruction than with either two-stage expander to implant or one-stage direct to implant reconstruction methods (17.3% compared to 4.5% and 4.1%) (15). Similarly, Ahn et al. reported a higher rate in NAC necrosis associated with transverse rectus abdominis myocutaneous (TRAM) flap reconstruction compared to expander-based reconstruction methods (14). This raises the question of whether it is the weight of the reconstruction, incision placement or traction injury are confounding factors that impair blood flow during surgery or the early postoperative period.
Perfusion of the NAC
The perfusion to the NAC is variable among patients. While the blood supply to the breast is reported to originate primarily from the internal thoracic, lateral thoracic, anterior intercostal and acromiothoracic arteries, studies have shown that there can be significant differences in the arterial branches to the NAC (16). The internal thoracic artery appears to be the predominant source via perforators in the first four intercostal spaces to supply the NAC (17).
Visualization of the circulatory anatomy of the skin is useful for the placement of incisions with the intent of avoiding division of a prominent vessel coursing to NAC. However, the use of real time assessments of perfusion to the NAC has been limited. There have been several imaging techniques utilized in the past to examine NAC perfusion intraoperatively. These include fluorescein dye angiography, and more recently, near infrared imaging coupled to indocyanine green (ICG) skin angiography. The latter has been routinely utilized by our group [SPY EliteTM imaging system (Novadaq Inc/Stryker)].
Four distinct arterial-arteriolar perfusion patterns to the NAC were defined: V1, originating from underlying breast tissue; V2, from the surrounding skin, V3, a combination of V1 and V2 and, V4, defined as diffuse capillary filling coursing from the skin periphery towards the NAC (18). Interestingly, the proportion of each of these patterns differed with the degree of breast ptosis (4). Specifically, 17% and 18% of patients with no ptosis or grade 1 ptosis had V1 patterns. Contrastingly, 34.5% and 86% of women with grade 2 and 3 ptosis exhibited V1 patterns, consistent with ischemic complications observed clinically. After devascularization, the V4 pattern was observed in 9%, 48% and 57% of patients with grades 1, 2, and 3 ptosis, respectively.
Definition of surgical delay
The principle of partially severing blood inflow as a means of stimulating a compensatory increase in perfusion to a tissue, is an old surgical technique (19). Typically, it is used to render random tissue flaps ischemic to induce an increase in vascularity before transfer weeks later. Historical accounts dating back to the 15th century described devascularization or partially dividing blood inflow of forearm flaps used for rhinoplasty reconstruction. Most preclinical and clinical studies focus on musculocutaneous flaps, which are inherently different from the devascularization of the breast skin envelope alone as described herein for staged nipple sparing mastectomies. However, the benefit of devascularization with respect to skin paddle survival in tissue flaps has been associated with an increase in angiogenic growth factors and cytokines in animal models (20).
The skin has a rich vascular network consisting of a superficial system coursing within the papillary dermis and a deep system that runs between the dermis and the subcutaneous fat. Choke vessels are small anastomosing branches connecting larger arterioles or venules thought to dilate with induced ischemia (21). Collectively, this research suggests that 1 to 2 weeks may be sufficient to induce vascular hypertrophy and possible ingrowth within the vascular network.
Devascularization technique
The technique entails dividing the plane along the superficial breast fascia below and beyond the NAC along with coring out of nipple ducts to exclude the presence of cancer. The dissection is carried out over a few centimeters beyond the areolar edge overlying skin and along the edges of the incision (Figure 1). The extent of this dissection depends on the location of the incisions, the size of the breast and whether a lumpectomy is performed concurrently. Variations on this technique have been reported. Palmieri and colleagues reported in 2005 an office-based procedure using sedation, subareolar infiltration with 20 mL carbocaine/epinephrine plus 80 mL saline and passing of laparoscopic electrified scissors from the periphery of the breast to dissect and divide along the subareolar plane (22). A modification of the approach involves placing a silicone sheet to prevent revascularization from the subjacent breast tissue via a transverse incision paralleling the inferior mammary fold (23).
Mastectomy and timing of delay
The concept of surgical delay surgery in the realm of nipple sparing mastectomies, was re-introduced as a first stage operative approach in 2012 (24). The authors describe undermining an area of approximately 5 cm below the NAC in 28 patients at risk for ischemic complications based on ptosis, biopsy scars or smoking. Nipple sparing mastectomies was delayed 7 to 21 days without loss of the NAC due to ischemia. Our group, as well as others, have reported favorable outcomes using the two-stage approach (4,25,26).
Delayed NSM preceded by devascularization of the NAC is applicable to patients with newly diagnosed breast cancer slated for upfront surgery or following neoadjuvant systemic therapy. Nodal staging and excision of index cancer should be incorporated with the initial devascularization procedure as well as sub-nipple biopsy. This provides an opportunity to obtain a more thorough pathological evaluation of the primary cancer, especially in cases of extensive ductal carcinoma in situ where occult invasion may be identified and would then necessitate a nodal staging procedure during the second stage. Additionally, the sub-nipple biopsy at this initial procedure can ensure a clear anterior nipple margin for oncologic safety in any patient where the sub-nipple tissue is questioned to be involved.
In our practice we prefer separate axillary incisions along the inferior aspect of the hair-bearing area of the axilla for sentinel node biopsy or axillary node dissection surgery. Not only does this approach provide better visibility of the axillary contents but minimizes retraction of skin. Additionally, if a patient requires completion axillary node dissection based on definitive pathology of sentinel nodes this can be undertaken during the second stage completion mastectomy.
Under routine circumstances, the timing between the two stages ranges from 3 to 6 weeks (4,27). Although infrequent, there are occurrences in which after the first stage of devascularization the histology and molecular profile of the index cancer changes, therefore requiring additional cancer management and delaying the timing for second stage. For example, from time to time a diagnostic core needle biopsy demonstrates ductal carcinoma in situ, but on surgical excision, a HER2-positive invasive component is discovered. Alternatively, more extensive nodal involvement may be identified with the nodal staging procedure at the time of initial surgery. Rather than postponing indicated chemotherapy and compromise oncologic care of the patient, the second stage NSM is deferred until systemic treatment is completed to not compromise the overall oncologic care of the patient. In these instances, we have delayed definitive mastectomy for more than 6 months with no difference in ability to complete the planned NSM.
Evaluation of skin perfusion patterns has improved our understanding of risk in this patient population and how these patients fare after a two-stage approach to NSM (4,17). Clinically significant epidermolysis was noted after devascularization in 63% of patients with a V1 pattern compared to 43% and 22% of V2 and V3 patterns, respectively (Figure 2). This suggests that V3 patterns harbor more robust vascular interconnections allowing them to compensate better from the loss of the breast tissue derived blood flow component (4). Following mastectomy, there was no nipple loss despite statistically non-significant differences in ischemic complications; 26% for V1, 7% V2 and 6% V3 (Figure 3). Finally, in a systematic review of 5 studies with 101 patients receiving NSM after devascularization, only 8.9% experienced partial NAC ischemia and none had total NAC necrosis (26). Altogether, these results demonstrate that devascularization and surgical delay is applicable to higher risk patients for NAC loss.
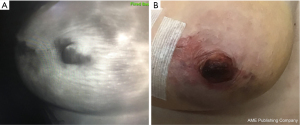
Macromastia and ptosis
Traditionally, very large or ptotic breasts are considered higher risk for NAC ischemic complications and necrosis. For these patients, reduction mammoplasty or mastopexy can be a first step toward achieving nipple-sparing mastectomy in a delayed fashion. One of the first to describe a staged approach in high-risk patients, Spear et al. described a series of 15 patients (24 breasts) with significant ptosis or macromastia who underwent a staged approach with a mastopexy or small reduction performed 3–4 weeks prior to completion mastectomy (27). Similarly, Martinovic et al. reported an average 17-day delay for patients who were characterized as “high risk” for nipple necrosis (28). The circumareolar area was de-epithelized and the time to completion mastectomy was 3.4 months. Other series described use of a modified Wise pattern incision for initial NSM with a later staged reconstruction (29,30). Momeni et al. reported on 122 breasts undergoing NSM with abdominal free flap reconstruction on average 16.9 weeks post-breast reduction surgery (31). NAC necrosis occurred in 6.6% of cases and partial loss in another 8.2%, all presenting among patients whose time interval to mastectomy ranged between 3 to 6 weeks.
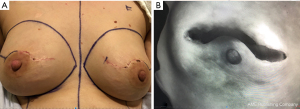
Devascularization can follow mastopexy procedures aimed at reducing a large skin envelope and repositioning of the NAC. In cancer cases, the lumpectomy and nodal staging procedure are performed with the reduction mammoplasty or mastopexy as a first stage. Given the common use of peri-areolar incisions in these operations, definitive NSM should be delayed 4 to 6 months, in order to allow for vessel ingrowth across the peri-areolar scar (Figure 4). In our practice, we have incorporated the devascularization step, 3 to 4 weeks prior to completion NSM, in order to further safeguard the viability of the NAC.
Neoadjuvant therapies
The use of neoadjuvant systemic therapies is on the rise. Surgical delay can also be applied to these patients. Pathological findings from surgical resection are critical as the burden of residual disease in the breast defines the need for additional systemic treatments as well as timing of adjuvant locoregional radiation. Again, it is conceivable to delay completion mastectomy and administer radiotherapy if adequate cancer surgery has been accomplished with either reduction mastopexy or initial devascularization procedures.
Contraindications to devascularization
Patients with large skin envelopes such as those with macromastia or grade 3 ptosis are not ideal candidates for devascularization alone prior to staged nipple sparing mastectomy. In these situations, completion mastectomy would likely lead to significant mastectomy skin envelope necrosis despite surgical delay. Our approach is to perform mastopexy or breast reduction mastopexy first with at least a 4 to 6 months delay to 2-stage nipple sparing mastectomy. Breast cancer operations should be performed concurrent with the breast reduction. Neoadjuvant or adjuvant therapies can be administered during this interim period, while allowing women to achieve the desired cosmetic results of nipple-areolar preserving mastectomy without delay of appropriate oncologic care.
Summary
Two-stage procedures inevitably are more costly but advantageous in that re-operations for ischemic complications after nipple-sparing mastectomies are reduced (23). Avoiding the loss of the NAC, the desired aesthetic aim of this type of mastectomy, should be viewed as an important goal, for it restores a normal appearance, and therefore impacts positively on quality of life. Staged devascularization allows more patients to achieve preservation of the nipple areola preservation, without compromising oncologic safety. We advocate this delayed technique for patients with risk factors for ischemia including those whose NAC perfusion is predominantly derived from the underlying breast tissue or who simply wish to optimize their outcomes with nipple sparing mastectomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dung Nguyen) for the series “Cutting-edge of Complex Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-111). The series “Cutting-edge of Complex Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. IW serves as an unpaid editorial board member of Annals of Breast Surgery from May 2020 to April 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wong SM, Chun YS, Sagara Y, et al. National Patterns of Breast Reconstruction and Nipple-Sparing Mastectomy for Breast Cancer, 2005-2015. Ann Surg Oncol 2019;26:3194-203. [Crossref] [PubMed]
- Headon HL, Kasem A, Mokbel K. The Oncological Safety of Nipple-Sparing Mastectomy: A Systematic Review of the Literature with a Pooled Analysis of 12,358 Procedures. Arch Plast Surg 2016;43:328-38. [Crossref] [PubMed]
- Agha RA, Omran Al Y, Wellstead G, et al. Systematic review of therapeutic nipple-sparing versus skin-sparing mastectomy. BJS Open 2018;3:135-45. [Crossref] [PubMed]
- Bertoni DM, Nguyen D, Rochlin D, et al. Protecting Nipple Perfusion by Devascularization and Surgical Delay in Patients at Risk for Ischemic Complications During Nipple-Sparing Mastectomies. Ann Surg Oncol 2016;23:2665-72. [Crossref] [PubMed]
- Frey JD, Salibian AA, Choi M, et al. Optimizing Outcomes in Nipple-sparing Mastectomy: Mastectomy Flap Thickness Is Not One Size Fits All. Plast Reconstr Surg Glob Open 2019;7:e2103. [Crossref] [PubMed]
- Chirappapha P, Petit JY, Rietjens M, et al. Nipple sparing mastectomy: does breast morphological factor related to necrotic complications? Plast Reconstr Surg Glob Open 2014;2:e99. [Crossref] [PubMed]
- Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol 2012;19:3402-9. [Crossref] [PubMed]
- Salibian AA, Frey JD, Bekisz JM, et al. Ischemic Complications after Nipple-sparing Mastectomy: Predictors of Reconstructive Failure in Implant-based Reconstruction and Implications for Decision-making. Plast Reconstr Surg Glob Open 2019;7:e2280. [Crossref] [PubMed]
- Paprottka FJ, Schlett CL, Luketina R, et al. Risk Factors for Complications after Skin-Sparing and Nipple-Sparing Mastectomy. Breast Care 2019;14:289-96. [Crossref] [PubMed]
- Margenthaler JA, Gan C, Yan Y, et al. Oncologic Safety and Outcomes in Patients Undergoing Nipple-Sparing Mastectomy. J Am Coll Surg 2020;230:535-41. [Crossref] [PubMed]
- Wijayanayagam A, Kumar AS, Foster RD, et al. Optimizing the total skin-sparing mastectomy. Arch Surg 2008;143:38-45. [Crossref] [PubMed]
- Tevlin R, Griffin M, Karin M, Wapnir I, et al. Impact of Incision Placement on Ischemic Complications in Microsurgical Breast Reconstruction. Plast Reconstr Surg 2021; In press.
- Odom EB, Parikh RP, Um G, et al. Nipple-Sparing Mastectomy Incisions for Cancer Extirpation Prospective Cohort Trial: Perfusion, Complications, and Patient Outcomes. Plast Reconstr Surg 2018;142:13-26. [Crossref] [PubMed]
- Ahn SJ, Woo TY, Lee DW, et al. Nipple-areolar complex ischemia and necrosis in nipple-sparing mastectomy. Eur J Surg Oncol 2018;44:1170-6. [Crossref] [PubMed]
- Endara M, Chen D, Verma K, et al. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg 2013;132:1043-54. [Crossref] [PubMed]
- van Deventer PV, Graewe FR. The blood supply of the breast revisited. Plast Reconstr Surg 2016;137:1388-97. [Crossref] [PubMed]
- Zhou M, Wapnir I, Kahn D. Blood supply to the nipple-areolar complex and intraoperative imaging of nipple perfusion patterns. In: Shiffman MA. editor. Nipple-Areolar Complex Reconstruction: Principles and Clinical Techniques. Springer International Publishing AG, 2018:55-65.
- Wapnir I, Dua M, Kieryn A, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol 2014;21:100-6. [Crossref] [PubMed]
- Ghali S, Butler PEM, Tepper OM, et al. Vascular delay revisited. Plast Reconstr Surg 2007;119:1735-44. [Crossref] [PubMed]
- Lineaweaver WC, Lei MP, Mustain W, et al. Vascular endothelium growth factor, surgical delay, and skin flap survival. Ann Surg 2004;239:866-73; discussion 873-5. [Crossref] [PubMed]
- Dhar SC, Taylor GI. The delay phenomenon: the story unfolds. Plast Reconstr Surg 1999;104:2079-91. [Crossref] [PubMed]
- Palmieri B, Baitchev G, Grappolini S, et al. Delayed nipple-sparing modified subcutaneous mastectomy: rationale and technique. Breast J 2005;11:173-8. [Crossref] [PubMed]
- Martinez CA, Reis SM, Boutros SG. The Nipple-Areola Preserving Mastectomy: The Value of Adding a Delay Procedure. Plast Reconstr Surg Glob Open 2016;4:e1098. [Crossref] [PubMed]
- Jensen JA, Lin JH, Kapoor N, et al. Surgical delay of the nipple-areolar complex: a powerful technique to maximize nipple viability following nipple-sparing mastectomy. Ann Surg Oncol 2012;19:3171-6. [Crossref] [PubMed]
- Dua MM, Bertoni DM, Nguyen D, et al. Using intraoperative laser angiography to safeguard nipple perfusion in nipple-sparing mastectomies. Gland Surg 2015;4:497-505. [PubMed]
- Karian LS, Therattil PJ, Wey PD, et al. Delay techniques for nipple-sparing mastectomy: A systematic review. J Plast Reconstr Aesthet Surg 2017;70:236-42. [Crossref] [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [Crossref] [PubMed]
- Martinovic ME, Pellicane JV, Blanchet NP. Surgical Delay of the Nipple-Areolar Complex in High-risk Nipple-sparing Mastectomy Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e760. [Crossref] [PubMed]
- Schwartz JD, Skowronksi PP. Improved Outcomes with Pedicled Nipple-sparing Mastectomies Using a New Surgical Delay: Mastectomy through Wise Incisions. Plast Reconstr Surg Glob Open 2017;5:e1259. [Crossref] [PubMed]
- Folli S, Mingozzi M, Curcio A, et al. Nipple-sparing mastectomy: an alternative technique for large ptotic breasts. J Am Coll Surg 2015;220:e65-9. [Crossref] [PubMed]
- Momeni A, Kanchwala S, Sbitany H. Oncoplastic Procedures in Preparation for Nipple-Sparing Mastectomy and Autologous Breast Reconstruction: Controlling the Breast Envelope. Plast Reconstr Surg 2020;145:914-20. [Crossref] [PubMed]
Cite this article as: Tsai J, Wapnir I. Protecting nipple-areolar complex perfusion by devascularization and surgical delay. Ann Breast Surg 2021;5:37.



