
Postmastectomy radiation: an evolution
History of postmastectomy radiation therapy (PMRT)
PMRT has been used in conjunction with surgery for decades to achieve locoregional control of breast cancer. It has been used to eliminate occult or microscopic disease present after surgery in attempt to reduce risk of locoregional recurrence and improve overall survival (OS). Early randomized trials demonstrated improvements in locoregional control, but not distant recurrence or OS particularly in the setting of suboptimal chemotherapy regimens and radiation protocols.
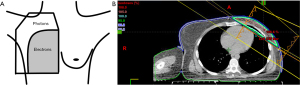
The inferiority of earlier chemotherapy regimens that did not demonstrate a survival benefit with PMRT is reflected in the high distant failure rates seen, approximately 40% (1,2). Additionally, antiquated two-dimensional (2D) radiation techniques and field design (Figure 1—diagram of reverse hockey stick plan) used in these early studies were based on X-ray imaging alone (3) and thus exposed large volumes of heart and lungs to radiation, undoubtedly contributing to equivalent or worse survival outcomes. Contemporary systemic therapy regimens have demonstrated better distant control and modern radiation techniques have minimized dose to heart, lungs, and contralateral breast.
In 2001, American Society of Clinical Oncology (ASCO) consensus guidelines for PMRT recommended routine use of PMRT in higher risk patients defined as presence of four or more positive nodes (N2) and patients with T3N0 or operable clinical stage III patients (i.e., tumors >5 cm in size and 1 or more nodes with metastases). The expert panel concluded there was insufficient evidence to support radiation in T1–T2 tumors with 1–3 involved nodes, and patients with other adverse pathologic features [e.g., lymphovascular invasion (LVSI), high grade]. Additionally, there was controversy in regards to inclusion of internal mammary nodes (IMNs) in the setting of regional nodal irradiation (4). More recent randomized and nonrandomized trials demonstrated a clear benefit of PMRT in patients with any nodes positive (5). Other studies also showed minimal toxicity with coverage of IMNs (6).
More recently, ASCO’s 2016 consensus guidelines updated recommendations to include patients with 1–3 positive axillary nodes. Expert panel also specified inclusion of supraclavicular (SCV), axillary, and IMNs in setting of regional nodal irradiation (7). Current National Comprehensive Cancer Network (NCCN) guidelines suggest PMRT be considered in setting of positive nodes, tumor >5 cm, positive or close margins (re-excision preferred), central/medial tumors, or tumors >2 cm with other high-risk features (i.e., young age or extensive LVSI). At the same time NCCN guidelines now reflect the more conservative recommendation of PMRT only for certain T3N0 tumors, with consideration based on high-risk features such as young age or extensive LVI (8). There are ongoing controversies about what populations can safely omit radiation and new controversies in terms of how to manage patients who receive neoadjuvant chemotherapy (NAC), which this review article will explore.
Rationale
Lymph node positive disease
Historically, radiotherapy was routinely delivered after mastectomy for women with involved lymph nodes based on early studies showing improvement in locoregional control (9,10). This practice was later brought into question, however, particularly when Cuzick and colleagues published a meta-analysis in 1987 of 7,941 patients who underwent mastectomy followed by observation vs. radiotherapy which showed an improvement in breast cancer cause-specific survival, but an excess of overall mortality in the radiotherapy cohort (11). The South-Eastern Cancer Study Group (SEG) subsequently found a trend towards reduction in locoregional recurrence, but no difference in survival in patients with four or more positive lymph nodes (12). The modern era has also seen drastic increases in utilization and efficacy of systemic therapy, and for a time PMRT fell out of favor, until more contemporary landmark trials from the Danish Breast Cancer Cooperative Group (DBCG) and British Columbia demonstrated reduction in locoregional recurrence and improved OS (Table 1).
Table 1
| Trial | N | Years | Follow-up | Menopausal status | Chemotherapy | OS | LRR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRM (%) | MRM + RT (%) | P value | MRM (%) | MRM + RT (%) | P value | |||||||
| British Columbia | 318 | 1979–1986 | 20 | Pre | CMF | 37 | 47 | 0.03 | 28 | 10 | 0.002 | |
| DBCG 82b | 1,708 | 1982–1989 | 15 | Pre | CMF | 45 | 54 | <0.001 | 32 | 9 | <0.001 | |
| DBCG 82c | 1,460 | 1982–1990 | 10 | Post | Tamoxifen* | 36 | 45 | 0.03 | 35 | 8 | <0.001 | |
*, tamoxifen required for only 1 year in DBCG 82c. PMRT, postmastectomy radiation therapy; OS, overall survival; LRR, locoregional recurrence; MRM, modified radical mastectomy; RT, radiation therapy; CMF, cyclophosphamide, methotrexate, and fluorouracil; Pre, premenopausal; Post, postmenopausal.
The DBCG 82b phase III trial enrolled 1,708 premenopausal women with high-risk pathologic features defined as one or more of the following: involvement of axillary lymph nodes, tumor size of >5 cm, invasion of cancer to skin or pectoral fascia. Patients were randomized to receive adjuvant radiotherapy plus cyclophosphamide, methotrexate, and fluorouracil (CMF) or CMF alone following total mastectomy and axillary node sampling (13). The Danish 82c trial randomized 1,375 high-risk postmenopausal women who underwent total mastectomy with partial axillary node dissection to tamoxifen alone or tamoxifen with PMRT (14). Radiation in both these trials was delivered to the chest wall and ipsilateral regional lymphatics (including SCV/infraclavicular, axillary, and IMNs at the first four intercostal spaces) to a dose of 48 Gy in 22 fractions or 50 Gy in 25 fractions. Both the DBCG 82b/c trials showed improvement in locoregional failure and OS (Table 1). A major criticism of both Danish trials was concern for inadequate axillary dissection and/or pathologic analysis given only a median of seven axillary nodes were removed. However, in a pooled reanalysis of a subset of patients in 82b/c trial in node positive patients who had eight or more lymph nodes dissected there was a significant reduction in 15-year loco-regional failure rate (27% to 4%, P<0.001; 51% to 10%, P<0.001) and survival benefit (57% to 48%, P=0.03; 21% vs. 12%, P=0.03) in patients with 1–3 positive nodes and patients with 4+ positive nodes (15). The British Columbia trial initiated in 1979 randomized node-positive premenopausal patients treated with modified radical mastectomy with axillary lymph node dissection of levels I and II (median of 11 lymph nodes resected) and adjuvant chemotherapy to PMRT or observation, and found statistically significant improvements in 20-year locoregional recurrence, disease free survival, and breast cancer specific survival, and OS (Table 1). Moreover, improvement in all survival outcomes with radiation in patients with one to three axillary lymph nodes involved was similar to patients with four or more lymph nodes involved (16,17). Radiation was delivered with 37.5 Gy in 16 fractions to chest wall, SCV, axillary, and bilateral IMNs. An additional collaborative analysis of prospective data from British Columbia and M.D. Anderson Cancer Center showed that in patients with 1–3 nodes a >0.20 involved nodal ratio regardless of number of nodes removed predicted risk of locoregional recurrence exceeding 20% (18). The results of these studies supported regional radiotherapy for high-risk patients who underwent mastectomy including tumor size of >5 cm, invasion of skin/pectoral fascia/chest wall, and any positive axillary lymph nodes.
In 2005, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis included 8,500 women who underwent mastectomy, axillary dissection and node positive disease enrolled in trials of radiotherapy (chest wall and regional lymph nodes). The meta-analysis found improvement in not only local recurrence at 5 years (23% vs. 6% with reduction 17%), but also breast cancer specific survival (60.1% vs. 54.7%). In consideration of less effective chemotherapy and outdated radiation techniques, EBCTCG concluded that there is a benefit in high-risk patients but PMRT remained controversial in low nodal burden populations (19).
In 2014, newer EBCTCG meta-analysis data demonstrated improvement in local control, overall control, and OS in patients with 1–3 nodes. This large meta-analysis of 22 randomized trial showed that for women with axillary dissection (including levels I and II and/or at least 10 axillary nodes removed) and four or more positive nodes, radiotherapy reduced locoregional recurrence [two-sided significance level (2P) <0.00001], overall recurrence [rate ratio (RR) 0.79, 95% CI: 0.69–0.90, 2P=0.0003], and breast cancer mortality (RR 0.87, 95% CI: 0.77–0.99, 2P=0.04) (5). Radiation reduced locoregional recurrence, overall recurrence and breast cancer specific mortality even for patients with one node positive. The EBCTCG reported that adjuvant radiation therapy (RT) following mastectomy and axillary dissection improved locoregional control at 10 years by 17.9% and reduced breast cancer mortality at 20 years by 8.1% in women with 1 to 3 positive lymph nodes.
Currently, the available randomized and non-randomized data suggests an approximately 60–70% decrease in the risk of loco-regional recurrence and a small decrease in cancer specific mortality with the addition of adjuvant PMRT in women with 1–3 nodes positive. Controversies remain related to radiation treatment recommendations in this setting including the relatively high local failure rate, up to 26–35% in the Danish and British Columbia studies and 20.3% in the EBCTCG report. However, the European Organization for Research and Treatment of Cancer (EORTC) 22922 trial looked at node positive and high-risk node negative patients with modern era techniques and still found a 1.2% reduction in locoregional recurrence and 3% increase in distant disease-free survival (DFS). These analyses show that many patients with one or more positive nodes benefit from PMRT. Whether this benefit extends to all patients, including hormone positive patients, is being investigated in MA39 (TAILOR RT) trial (20).
A summary table of trials supporting the use of PMRT in lymph node positive patients can be found in Table 2.
Table 2
| Trial | N | Years | Follow-up (years) | BCM | Any recurrence | LRR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No RT (%) | RT (%) | P value | No RT (%) | RT (%) | P value | No RT (%) | RT (%) | P value | ||||||
| EBCTG meta-analysis (pN+) | 3,131 | 1964–1986 | 10 | 66.4 | 58.3 | 0.001 | 62.5 | 51.9 | <0.00001 | 26.0 | 8.1 | <0.00001 | ||
| EORTC 22922* | 4,004 | 1996–2004 | 10.9 | 14.4 | 12.5 | 0.02 | 22.9 | 19.4 | 0.015 | 9.5 | 8.3 | – | ||
Any recurrence defined as any first breast recurrence which includes local/regional recurrence, distant progression, second ipsilateral breast cancer or death due to breast cancer. *, in EORTC 22922, only 955 patients underwent mastectomy and a separate subanalysis of outcomes not performed. PMRT, postmastectomy radiation therapy; BCM, breast cancer mortality; LRR, locoregional recurrence; RT, radiation therapy; DNR, did not report.
Lymph node negative disease
Retrospective analysis of DBCG 82b/c and British Columbia trials have demonstrated certain patient populations that have higher local recurrence rates and may therefore derive more benefit from supplemental external beam RT (21). High-risk patients were defined as having involvement of axillary lymph nodes, tumor size of >5 cm, invasion of cancer to skin or pectoral fascia in these trials. A multivariate analysis found size of primary tumor, frequency and number of positive lymph nodes, histopathologic grade, use of radiotherapy, and age were all significant predictors of outcome (recurrence or death) (15). In an observational study of 94 patients with pT1–2 pN0 with positive surgical margins there was a trend towards higher locoregional relapse rates (~20%) in patients who did not receive PMRT and had at least one of the following additional clinicopathologic factors: age ≤50 years, T2 tumor size, high-grade disease, or LVSI (22). Another randomized controlled trial found improved recurrence-free survival and OS in stage I–II triple negative breast cancer patients who received total mastectomy and partial axillary dissection followed by adjuvant radiation and chemotherapy compared to chemotherapy alone (23).
There is also a retrospective analysis of 1,136 node-negative T1–2 breast cancer patients treated with total mastectomy and axillary node dissection without PMRT at Massachusetts General Hospital that found tumor size ≥2 cm, close margins defined as ≤2 mm or positive margins, LVSI, age ≤50, and omission of systemic therapy were associated with higher locoregional recurrence risk (LRR) (24). The 10-year cumulative incidence of LRR for patients with no risk factors was 2.0% vs. 19.7% in patients with three or more risk factors.
More recent studies with modern radiation techniques and systemic therapy including MA-20 and EORTC 22922 have also supported adjuvant breast/chest wall and regional node irradiation (medial SCV, IMN, apical axillary nodes). MA-20 only included patients who underwent breast-conserving surgery and axillary lymph node dissection and were node positive as well as high-risk node negative patients. High-risk features were defined as tumor measuring ≥5 or ≥2 cm with fewer than ten axillary nodes removed and at least one of the following: grade 3, ER negative, or LVSI. The investigators found that receipt of regional nodal irradiation conferred a DFS benefit (25). Although not a PMRT study per se, the results are nonetheless important to consider regarding the potential benefit of regional nodal RT in postmastectomy patients, since the surgical treatment of nodal regions in question may not differ significantly between breast conservation and mastectomy. The EORTC 22922 trial included 24% patients who underwent total mastectomy and axillary lymph node dissection followed by radiation +/− regional nodal irradiation was comprised of node negative patient with high-risk features (central/medial tumors) and node positive patients. At 10 years, additional regional nodal irradiation resulted in significantly improved breast cancer mortality rate (12.5 vs. 14.4%, P=0.01), improved DFS (72% vs. 69%, P=0.04) and a trend toward improved OS (82.3 vs. 80.7%, P=0.06) (26).
The identification of high-risk node negative patient populations continues to evolve, but PMRT should certainly be considered in central/medial tumors, tumors >5 cm, T1–2 disease with other adverse clinicopathologic features (e.g., age ≤50 years, high-grade disease, triple negative histology, or LVSI), and positive margins.
A summary table of trials supporting the use of PMRT in lymph node positive patients can be found in Table 3.
Table 3
| Trial | N | Years | Follow-up (years) | DFS | OS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No RT (%) | RT (%) | P value | No RT (%) | RT | P value | |||||
| EORTC 22922 | 4,004 | 1996–2004 | 10.9 | 72.1 | 69.1 | 0.04 | 80.7 | 82.3 | 0.06 | |
| MA-20* | 1,832 | 2000–2007 | 9.5 | 77.0 | 82.0 | 0.01 | 81.8 | 82.8 | 0.11 | |
| Fourth Military Medical University (China)** | 681 | 2001–2006 | 7.2 | 74.6 | 88.3 | 0.02 | 78.7 | 90.4 | 0.03 | |
*, MA-20 was not a PMRT trial, but still informs radiation in lymph node negative patients. **, Fourth Military Medical University investigated triple negative stage I–II breast cancer patients with 86.1% being node negative. RT, radiation therapy; DFS, disease-free survival; OS, overall survival.
Impact of NAC
There are currently no randomized clinical trial data to guide management of patients who receive NAC. We know that pathologic complete response (pCR) is prognostic for improved OS (27). There are open trials including National Surgical Adjuvant Breast and Bowel Project (NSABP) B51 which aims to evaluate role of regional radiotherapy in patients with pCR.
In a combined analysis of NSABP B18 and B27, Mamounas and colleagues examined locoregional recurrence after NAC in patients who did not receive regional radiotherapy. In the mastectomy cohort, 10-year cumulative incidence of locoregional recurrence was 12.6%. On multivariate analysis, there were five factors associated with LRR including: age, clinical tumor size >5 cm, clinically positive nodes, pCR in nodes but no pCR in breast, and pathologically positive nodes after NAC (28). In a pooled analysis from the Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC), tumor subtype and pathologic response were independent predictors of locoregional recurrence. Specifically, triple negative patients had three times the 5-year cumulative incidence of LRR compared to hormone receptor positive, grade 1–2 patients. In the phase III EORTC 10994 trial, multivariate analysis of LRR following neoadjuvant therapy found luminal A subtypes had the lowest rate of recurrence compared to triple negative and patients achieving pCR had the lowest rate of recurrence compared to those with residual disease in breast and nodes after NAC (29). Another retrospective analysis at M.D. Anderson found patients with clinical T3N0 disease treated with NAC and mastectomy, but without PMRT had significantly elevated risk of LRR when compared to patients who received PMRT (30).
The role of PMRT is controversial for patients with clinical lymph node positivity who subsequently achieve a complete response in the axilla, and multiple studies have failed to demonstrate a significant benefit in clinical stage II (cT2–T3, cN1) patients with pCR after NAC (31,32). In their 2012 study, Fowble and colleagues quantified the risk of recurrence in women who underwent NAC followed by mastectomy but who did not undergo PMRT (33). In this study, women with pCR following NAC had a low rate of local regional recurrence (3%). While this showed no recurrences (0%) in women with HR+/Her2−/HR−/Her2−/HR−/Her2+ disease and a higher risk for recurrence among women with HR+/Her2+ (14%) disease, the overall numbers from this study were small and the role of RT in this setting remains uncertain.
Huang and colleagues at M.D. Anderson Cancer Center performed a meta-analysis of six prospective NAC trials and found post-mastectomy radiation and regional nodal irradiation improved 10-year cause specific survival in locally advanced breast cancer including: clinical stage IIIB or higher, T4, N2–3, four or more pathologically positive nodes (ypN2). Locoregional recurrence rate was reduced from 22% to 11% with PMRT and patients with cT3–4 tumors, stage IIB or greater, ypT2 or greater, and ypN2 or greater demonstrating significant benefit (34).
Current indications for consideration of PMRT in setting of NAC include axillary node involvement after systemic therapy (ypN+), residual breast disease (ypT+), cT3–4, or above cN2–3 disease. There is less evidence to guide management of patients with cN1 disease that become ypN0 and stage I–II (except T3N0) after chemotherapy. NSABP B51 RTOG 1304 seeks to elucidate which of these patients benefit from radiation. Observation may be indicated for patients with clinically small volume of disease, pCR in breast and nodes or residual T1 luminal subtype and older age (35).
Treatment techniques
Standard fields
There has been an immense evolution of modern radiation techniques transitioning from two-dimensional field to three-dimensional computed tomography (CT) planning. Standard post-mastectomy radiation is typically delivered using photons with forward planning technique termed 3D conformal radiation therapy (3DCRT). Chest wall irradiation alone typically involves two opposed tangential fields. In order to treat chest wall and regional nodes 3 to 4 fields are typically used: two opposed tangential fields, one anterior oblique SCV field and often a posterior/oblique field which can increase deep SCV & axillary coverage. Sometimes a separate anterior IMN field is also added, when clinically appropriate (see IMN section below). Different beam angles, field sizes, beam energies, and weighting are then employed to create an optimal treatment plan. Deep inspiratory breath-hold (DIBH) is a technique whereby patients are treated during inspiration and is routinely used to minimize heart dose, particularly in left sided breast cancer, as well as ipsilateral and total lung dose. Pulmonary and cardiac toxicity are minimized with shielding by multi-leaf collimators. A chest wall bolus comprised of tissue-equivalent material is often used to increase skin dose in higher risk patients. A 0.5–1-cm bolus may be applied every day or every other day, for example.
Conventional dose and fractionation for PMRT is 50–50.4 Gy in 1.8–2.0 Gy per fraction in 25–28 fractions to the chest wall. A chest wall scar boost of 10 to 16 gray in 4–8 fractions may also be employed. Although no randomized data is available to guide decisions about chest wall boost, it should certainly be considered in cases of inflammatory breast cancer or close/positive margins. Generally, clinicians aim for at least 90–95% of prescription dose coverage or around 45–50 Gy to regional nodes. Hypofractionated protocols are under investigation as described below.
IMNs
The DBCG IMN prospective cohort of 3,089 node positive breast cancer patients received radiation to the chest wall, SCV, undissected axilla and right sided cases also received IMN radiation (whereas left sided cases did not receive IMN coverage). They found at 8 years improved breast cancer mortality and OS with IMN irradiation and trend toward reduction in distant recurrence (6). More widespread coverage of IMNs has been supported by several large randomized trials with minimal toxicity. A single institution retrospective analysis 169 women treated with chest wall/breast, SCV, axilla, and IMNs. IMN radiation did not lead to unacceptable heart and lung doses with use of 3DCRT (36). Based on EORTC 22922 trial results, patients with central/medial tumors or axillary nodes should receive IMN radiation (26).
Modified wide tangent fields can be used in which opposed tangent fields are widened superiorly to include to cover age of three intercostal spaces that contain the IMNs. Alternatively, a separate anterior field can be added with either electrons or photons.
IMRT/protons
Intensity-modulated radiation therapy (IMRT) is considered when there are concerns about heart or lung dose or constraints are not met with conventional 3DCRT. IMRT also utilizes photons but involves more beams and an inverse planning technique whereby goals of coverage and dose constraints are entered and a treatment planning system creates an optimal plan. A potential downside of this technique is a larger area of low-dose radiation delivered to other parts of the body than with standard 3DCRT.
The use of proton beam radiation is currently under investigation. Protons have unique dose deposition characteristics in that they deposit maximum dose at specific depths in tissue (Bragg peak), minimizing entry and exit dose. The RADCOMP Consortium trial is comparing photon to proton therapy in nonmetastatic patients receiving breast/chest wall and regional node irradiation (37).
Hypofractionation
In breast conservation therapy, hypofractionated whole breast irradiation has become the standard of care (38). In setting of mastectomy, hypofractionation is still under investigation. Peking Union Medical College in Beijing, China evaluated 811 patients with T3–4 or ≥4 nodes who underwent mastectomy without reconstruction were randomized to receive conventional fractionated PMRT (chest wall, undissected axilla, SCV and IMNs) to dose of 50 Gy in 25 fractions vs. hypofractionated PMRT to a dose of 43.5 Gy in 15 fractions. This study showed no differences in LRR, DFS, OS, or toxicity (39). UK Fast Forward also demonstrated noninferiority of 26 Gy in 5 fractions compared to 40 Gy in 15 fractions to chest wall alone, but only 7% of the study cohort underwent mastectomy (40). The START A and B trials also showed favorable outcomes with minimal toxicity of 39 Gy in 13 fractions, 41.6 Gy in 13 fractions, and 40 Gy in 15 fractions, but again only 15% of this patient cohort received hypofractionated chest wall irradiation (41). The SUPREMO trial is ongoing and seeks to evaluate PMRT (chest wall only) in T1–2 and 1–3 nodes positive, T2N0 grade 3, or T2N0 with LVSI with choice of 40 Gy in 15 fractions, 45 Gy in 20 fractions, or 50 Gy in 25 fractions (42). Two-year follow-up results show minimal toxicity in published early analysis (43).
Of the above studies, the Beijing study is the only one to provide randomized evidence that hypofractionation of chest wall and regional nodes is noninferior in non-reconstructed patients. We await the results of the Alliance RT Charm trial that will evaluate hypofractionated PMRT in reconstructed patients (44).
For patients with prior breast augmentation, there was a recent study published by Tadros et al. to evaluate feasibility of hypofractionated in setting of prior breast augmentation. Seventy-one patients were retrospectively analyzed. In this group, 39.4% of patients were treated with conventionally fractionated RT, 54.9% of patients were treated with hypofractionated RT, 84.5% of patients were not treated with a third radiation field, 81.7% of patients received a breast boost, and 87.3% of patients underwent a retropectoral breast augmentation procedure. Of these 71 patients, physician rated assessment after completing RT was “Excellent” in 60.6% of cases and “Good” in 26.8% of cases. Additionally, 25.4% of patients developed a new or worsening of breast contracture after therapy and 12.7% were referred to plastic surgery for a surgical revision. There were no instances of implant-loss. On univariate analysis, implant location, time from implant to diagnosis, RT type, RT boost, body mass index (BMI), and tumor size were not associated with new or worse contracture. However, limitations of this study are its relatively short mean follow-up of 1.9 years (45). Based on retrospective studies, hypofractionation in setting of reconstruction and prior breast augmentation appears to be noninferior to standard fractionation with low toxicity, but more data is necessary in order to make definitive recommendations.
Treatment side effects
Radiation side effects are typically categorized as acute or late, with common acute effects of PMRT including dermatitis and fatigue that typically resolve in the weeks following completion. Because so many breast cancer patients go on to become long term survivors, a detailed discussion of more serious or long term (late) effects is also necessary. Lung toxicity can range from asymptomatic fibrosis within the treatment beam path or, rarely, pneumonitis. Fortunately, modern planning techniques allow for strict constraints on lung dose leading to reduced complication rates (46). Radiation-induced cardiac toxicity was a big issue historically, and a 7.4% increase in cardiac events has been reported for each 1 Gy mean heart dose (47). With CT-based planning it is now much easier to ensure that the heart is not directly within the radiation field. Notably, coincident with widespread adoption of 3DCRT was increased use of cardiotoxic systemic therapy regimens including adriamycin, taxanes, trastuzumab, and pertuzumab. Lymphedema has plagued many recipients of PMRT, but interestingly a 2020 study found on multivariate analysis that the primary risk stems from the axillary surgery itself rather than adjuvant treatment (48). Finally, cosmesis can always be impacted by RT, a particular concern for patients undergoing reconstruction. Clinicians should discuss the potential impact of radiation on reconstruction with these patients as it may result in contracture, fibrosis, impaired wound healing, reconstructive failure, pain and breast asymmetry (49-52). In a retrospective study of patients receiving flap reconstruction, 31.6% of patients who received radiation after reconstruction (51). While there are certainly advantages to direct-to-implant procedure, these patients have higher incidence of complications in setting of PMRT. In a study by Roostaeian and colleagues, radiation after immediate implant reconstructions had higher need for revision and lower aesthetic outcome compared to two-stage implant reconstruction (52). According to current NCCN guidelines, immediate autologous reconstruction and implant is not preferred if there is plan for radiation following mastectomy (8). As a result, tissue expanders are often placed at time of surgery and permanent implant placement or delayed autologous tissue breast reconstruction is usually deferred for approximately six months following completion of RT.
Ongoing questions and future directions
There are ongoing studies exploring omission of radiation in low-risk patients. Despite evidence from EBCTCG that T1–2 patients with 1–3 axillary nodes demonstrate a LRR reduction from PMRT, these trials reported higher local recurrence rates (21%) compared to more modern trials (4–10%) using contemporary systemic therapies and standard axillary dissection (≥10 removed nodes), which limits generalizability of these results to all patients (7). Omission of regional radiotherapy (chest wall and regional nodes) in low-risk node positive patients (i.e., age >50, ER+ and/or PR+, HER2-negative, 1–3 positive axillary nodes, oncotype DX recurrence score ≤18) is currently being investigated in CCTG MA-39 Tailor RT phase III randomized trial (20). The SUPREMO trial will also evaluate PMRT in high-risk node negative disease and early-stage disease with one to three positive nodes (43). Clinical trials will help determine whether these lower-risk patients benefit from PMRT with modern systemic therapy and surgery.
In view of increasing utilization of NAC, there remain many unanswered questions regarding role of PMRT. The NRG NSABP B-51/RTOG 1304 phase III randomized trial is evaluating benefit of regional radiotherapy in setting of upfront clinical T1–3N1 disease with no pathologic nodes positive (ypN0) after NAC with or without HER2 directed therapies and surgery. These trials will help elucidate whether radiotherapy can be omitted in favorable risk cohorts and patients with complete pathologic response to NAC. A few trials including RT Charm and SUPREMO are currently underway to evaluate whether more hypofractionated regimens can be employed for postmastectomy (chest wall and regional nodal) radiation (42,44). Additionally, incorporation of genetic and molecular information into clinical practice is under investigation. For example, I-SPY 2 uses MammaPrint to stratify patients into various systemic therapy arms (53). Several studies are also investigating more modern radiation techniques such as IMRT and proton therapy. There is certainly still controversy in role of PMRT in certain subgroups of patients, but it remains true that both surgery and radiation are vital in locoregional control.
Pearls
- PMRT is associated with reduction in locoregional recurrence and improved OS in high-risk patients including those with positive lymph nodes, tumor greater than 5 cm, skin or chest wall invasion, and node negative patients with high-risk features: ER−, triple negative histology, grade 3, LVSI, central/medial tumors, young age (≤50 years or premenopausal status), close or positive margins.
- After NAC, PMRT is likely appropriate if cT3–4, cN2–3, or residual nodal disease (ypN+) is present. Full indications for PMRT after NAC are not fully established, however, and decision-making should be shared in a multidisciplinary capacity allowing for recommendations to be individualized based on unique clinicopathologic features. In the absence of randomized evidence, PMRT should be considered in certain stage I–II (cT2, cN1) patients with high-risk factors: young age, high grade histology, triple negative receptor status, LVSI, close/positive margins, or other adverse pathologic features.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (William Small Jr. and Parul Barry) for the series “Advancements and Opportunities for Breast Irradiation” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-120). The series “Advancements and Opportunities for Breast Irradiation” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B, Slack NH, Cavanaugh PJ, et al. Postoperative radiotherapy in the treatment of breast cancer: results of the NSABP clinical trial. Ann Surg 1970;172:711-32. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Pezner RD, Lipsett JA, Forell B, et al. The reverse hockey stick technique: postmastectomy radiation therapy for breast cancer patients with locally advanced tumor presentation or extensive loco-regional recurrence. Int J Radiat Oncol Biol Phys 1989;17:191-7. [Crossref] [PubMed]
- Recht A, Edge SB, Solin LJ, et al. Postmastectomyradiotherapy: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1539-69. [Crossref] [PubMed]
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. Erratum in: Lancet 2014 Nov 22;384(9957):1848. [Crossref] [PubMed]
- Thorsen LBJ, Offersen BV, Danø H, et al. DBCG-IMN: A Population-Based Cohort Study on the Effect of Internal Mammary Node Irradiation in Early Node-Positive Breast Cancer. J Clin Oncol 2016;34:314-20. [Crossref] [PubMed]
- Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J Clin Oncol 2016;34:4431-42. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer (Version 6.2029). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed May 10, 2020.
- Weichselbaum RR, Marck A, Hellman S. The role of postoperative irradiation in carcinoma of the breast. Cancer 1976;37:2682-90. [Crossref] [PubMed]
- Brady LW, Fletcher GH, Levitt SH. Cancer of the breast: The role of radiation therapy after mastectomy. Cancer 1977;39:2868-74. [Crossref] [PubMed]
- Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol 1994;12:447-53. [Crossref] [PubMed]
- Vélez-García E, Carpenter JT Jr, Moore M, et al. Postsurgical adjuvant chemotherapy with or without radiotherapy in women with breast cancer and positive axillary nodes: a South-Eastern Cancer Study Group (SEG) Trial. Eur J Cancer 1992;28A:1833-7. [Crossref] [PubMed]
- Overgaard M, Hansen PS, Overgaard J, et al. Postoperative Radiotherapy in High-Risk Premenopausal Women with Breast Cancer Who Receive Adjuvant Chemotherapy. N Engl J Med 1997;337:949-55. [Crossref] [PubMed]
- Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641-8. [Crossref] [PubMed]
- Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol 2007;82:247-53. [Crossref] [PubMed]
- Ragaz J, Jackson SM, Le N, et al. Adjuvant Radiotherapy and Chemotherapy in Node-Positive Premenopausal Women with Breast Cancer. N Engl J Med 1997;337:956-62. [Crossref] [PubMed]
- Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional Radiation Therapy in Patients With High-Risk Breast Cancer Receiving Adjuvant Chemotherapy: 20-Year Results of the British Columbia Randomized Trial. J Natl Cancer Inst 2005;97:116-26. [Crossref] [PubMed]
- Truong PT, Woodward WA, Thames HD, et al. The ratio of positive to excised nodes identifies high-risk subsets and reduces inter-institutional differences in locoregional recurrence risk estimates in breast cancer patients with 1-3 positive nodes: an analysis of prospective data from British Columbia and the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys 2007;68:59-65. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Canadian Cancer Trials Group. Regional Radiotherapy in Biomarker Low Risk Node Positive Breast Cancer (TAILOR RT). Available online: https://clinicaltrials.gov/ct2/show/NCT03488693. NLM identifier: NCT03488693. Accessed September 18, 2020.
- Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol 2008;26:1419-26. [Crossref] [PubMed]
- Truong PT, Olivotto IA, Speers CH, et al. A positive margin is not always an indication for radiotherapy after mastectomy in early breast cancer. Int J Radiat Oncol Biol Phys 2004;58:797-804. [Crossref] [PubMed]
- Wang J, Shi M, Ling R, et al. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: A prospective randomized controlled multi-center trial. Radiother Oncol 2011;100:200-4. [Crossref] [PubMed]
- Jagsi R, Raad RA, Goldberg S, et al. Locoregional recurrence rates and prognostic factors for failure in node-negative patients treated with mastectomy: Implications for postmastectomy radiation. Int J Radiat Oncol Biol Phys 2005;62:1035-9. [Crossref] [PubMed]
- Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015;373:307-16. [Crossref] [PubMed]
- Poortmans PM, Collette S, Kirkove C, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med 2015;373:317-27. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. Erratum in: Lancet 2019 Mar 9;393(10175):986. [Crossref] [PubMed]
- Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 2012;30:3960-6. [Crossref] [PubMed]
- Gillon P, Touati N, Breton-Callu C, et al. Factors predictive of locoregional recurrence following neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancer: An analysis of the EORTC 10994/BIG 1-00 study. Eur J Cancer 2017;79:226-34. [Crossref] [PubMed]
- Nagar H, Mittendorf EA, Strom EA, et al. Local-regional recurrence with and without radiation therapy after neoadjuvant chemotherapy and mastectomy for clinically staged T3N0 breast cancer. Int J Radiat Oncol Biol Phys 2011;81:782-7. [Crossref] [PubMed]
- Daveau C, Stevens D, Brain E, et al. Is regional lymph node irradiation necessary in stage II to III breast cancer patients with negative pathologic node status after neoadjuvant chemotherapy? Int J Radiat Oncol Biol Phys 2010;78:337-42. [Crossref] [PubMed]
- Le Scodan R, Selz J, Stevens D, et al. Radiotherapy for stage II and stage III breast cancer patients with negative lymph nodes after preoperative chemotherapy and mastectomy. Int J Radiat Oncol Biol Phys 2012;82:e1-7. [Crossref] [PubMed]
- Fowble BL, Einck JP, Kim DN, et al. Role of Postmastectomy Radiation After Neoadjuvant Chemotherapy in Stage II-III Breast Cancer. Int J Radiat Oncol Biol Phys 2012;83:494-503. [Crossref] [PubMed]
- Huang EH, Tucker SL, Strom EA, et al. Postmastectomy Radiation Improves Local-Regional Control and Survival for Selected Patients With Locally Advanced Breast Cancer Treated With Neoadjuvant Chemotherapy and Mastectomy. J Clin Oncol 2004;22:4691-9. [Crossref] [PubMed]
- NSABP Foundation Inc. Standard or Comprehensive Radiation Therapy in Treating Patients with Early-Stage Breast Cancer Previously Treated with Chemotherapy and Surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT01872975. NLM identifier: NCT01872975. Accessed September 19, 2020.
- Bazan J, DiCostanzo D, Kuhn K, et al. Likelihood of unacceptable normal tissue doses in breast cancer patients undergoing regional nodal irradiation in routine clinical practice. Pract Radiat Oncol 2017;7:154-60. [Crossref] [PubMed]
- Bekelman JE, Lu H, Pugh S, et al. RadComp (Radiotherapy Comparative Effectiveness Consortium). Pragmatic randomised clinical trial of proton versus photon therapy for patients with non-metastatic breast cancer: the Radiotherapy Comparative Effectiveness (RadComp) Consortium trial protocol. BMJ Open 2019;9:e025556. [Crossref] [PubMed]
- Smith BD, Bellon JR, Blitzblau R, et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 2018;8:145-52. [Crossref] [PubMed]
- Wang SL, Fang H, Song YW, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol 2019;20:352-60. [Crossref] [PubMed]
- Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020;395:1613-26. [Crossref] [PubMed]
- START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371:1098-107. [Crossref] [PubMed]
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-94. [Crossref] [PubMed]
- Velikova G, Williams LJ, Willis S, et al. Quality of life after postmastectomy radiotherapy in patients with intermediate-risk breast cancer (SUPREMO): 2-year follow-up results of a randomised controlled trial. Lancet Oncol 2018;19:1516-29. [Crossref] [PubMed]
- Alliance for Clinical Trials in Oncology. Hypofractionated Radiation Therapy After Mastectomy in Preventing Recurrence in Patients with Stage IIa-IIIa Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03414970. NLM identifier: NCT03414970. Accessed September 19, 2019.
- Tadros AB, Moo TA, Zabor EC, et al. Feasibility of Breast-Conservation Therapy and Hypofractionated Radiation in the Setting of Prior Breast Augmentation. Pract Radiat Oncol 2020;10:e357-62. [Crossref] [PubMed]
- Blom Goldman U, Wennberg B, Svane G, et al. Reduction of radiation pneumonitis by V20-constraints in breast cancer. Radiat Oncol 2010;5:99. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Naoum GE, Roberts S, Brunelle CL, et al. Quantifying the Impact of Axillary Surgery and Nodal Irradiation on Breast Cancer-Related Lymphedema and Local Tumor Control: Long-Term Results From a Prospective Screening Trial. J Clin Oncol 2020;38:3430-8. [Crossref] [PubMed]
- Tallet AV, Salem N, Moutardier V, et al. Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant: complications and esthetic results. Int J Radiat Oncol Biol Phys 2003;57:136-42. [Crossref] [PubMed]
- Classen J, Nitzsche S, Wallwiener D, et al. Fibrotic changes after postmastectomy radiotherapy and reconstructive surgery in breast cancer. A retrospective analysis in 109 patients. Strahlenther Onkol 2010;186:630. [Crossref] [PubMed]
- Williams JK, Carlson GW, Bostwick J 3rd, et al. The effects of radiation treatment after TRAM flap breast reconstruction. Plast Reconstr Surg 1997;100:1153-60. [Crossref] [PubMed]
- Roostaeian J, Pavone L, Da Lio A, et al. Immediate placement of implants in breast reconstruction: patient selection and outcomes. Plast Reconstr Surg 2011;127:1407-16. [Crossref] [PubMed]
- Park JW, Liu MC, Yee D, et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med 2016;375:11-22. [Crossref] [PubMed]
Cite this article as: Jones BM, Osborn VW. Postmastectomy radiation: an evolution. Ann Breast Surg 2021;5:38.

