Acellular dermal matrix used for lumpectomy cavity volume replacement mimicking as breast cancer recurrence: a case report
Introduction
Acellular dermal matrix (ADM) has been used as a soft tissue replacement since its introduction in 1994 (1). In breast surgery, ADM is used in more than 75% of immediate tissue expander reconstruction procedures to support the implant (2).
The role of ADM in these procedures is only to support the implant and provides no volume replacement. Yet, a recently published prospective study including 120 patients described filling the defects during breast conservation surgery (BCS) exclusively with diced ADM and demonstrated satisfactory short-term results for selected patients (3).
The literature on imaging features of ADM remains limited and imaging diagnosis of ADM remains largely based on clinical history and short-term follow-up (4).
Therefore, ADM has increasingly become a differential consideration in diagnostic imaging, following reconstructive breast surgeries. When such patients present with a palpable area of concern, the challenges encountered in differentiating imaging features of ADM from recurrent or developing disease are similar to those faced with fat necrosis or suture granulomas (5-8).
This report describes the radiological findings in a case of ipsilateral breast mass after 6 months from BCS that was initially interpreted as a breast mass suspicious for recurrence. This case is one which highlights clinical presentation and radiological features of the new technique ADM in breast reconstruction following lumpectomy and elucidate imaging findings.
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/abs-20-93).
Case presentation
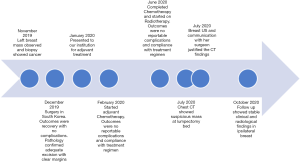
The patient is a 57-year-old female, her past medical history is significant for chronic iron deficiency anemia, hypertension and dyslipidemia and her family history is negative for malignancies. She was diagnosed with early left breast cancer in November 2019 and underwent left breast lumpectomy and sentinel lymph node biopsy in December 2019 in Seoul, South Korea. Final histopathology revealed a low grade Invasive Ductal Carcinoma of the left breast of TNM stage pT1c N0 M0, Estrogen/Progesterone receptors were positive, HER-2/neu was negative and the resection margins were negative for malignancy or atypia.
She presented to Cleveland Clinic Abu Dhabi (CCAD) in the United Arab Emirates in January 2020 for adjuvant treatment. She had few details regarding her operative care at presentation.
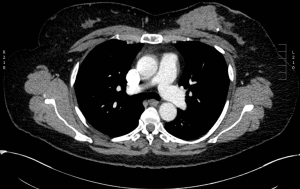
Genomic profiling revealed high risk of recurrence, accordingly, she received four cycles of adjuvant Doxorubicin (60 mg/m2/dose intravenous every 21 days) and Cyclophosphamide (600 mg/m2/dose intravenous every 21 days) followed by four cycles of Paclitaxel (175 mg/m2/dose intravenous every 21 days), then maintained on Letrozole (2.5 mg daily). During her surveillance work up six month after initial treatment, chest computed tomography (CT) showed a 5.7 cm × 3.5 cm × 2.7 cm mass with a lobulated margin in the vicinity of the resected tumor in left breast and residual disease or recurrence could not be ruled out (Figure 1).
Clinical examination did not reveal any suspicious ipsilateral breast mass, or pathological skin/nipple changes. Focused left breast ultrasound (US) at the same time showed a 7.7 cm × 2 cm × 4.4 cm artificial appearing mass which has a smoothly marginated border and swirling debris at the site of the previously resected malignancy (Figure 2).

In light of this, we were in contact with her local surgeon in Seoul and learned that in fact patient had undergone the new procedure of lumpectomy cavity volume replacement with diced ADM. The imaging findings were then interpreted as being consistent with this procedure. Follow up 3 months later unveiled stable clinical and radiological findings in ipsilateral breast, and the plan is to follow her up every 6 months (Figure 3).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
ADMs are soft tissue matrix grafts created by a process that results in decellularization but leaves the extracellular matrix intact. This matrix provides a scaffold upon and within which the patient’s own cells can repopulate and revascularize the implanted tissue. Its utility has been demonstrated in various reconstructive techniques, particularly in burn, abdominal wall, and breast reconstruction (1,9,10). Another advantage of ADM is that it is composed of cells that are resistant to radiation and have already been applied irradiation (11).
ADM can be classified as human, porcine, or bovine ADM, depending on the source (12).
When using ADM for reconstruction in breast surgery, the most common short-term complications are seroma formation (11%) and inflammation without infection which is called Red Breast Syndrome (RBS) in (2–6%) of cases (13-15). RBS is a delayed-type hypersensitivity reaction that rarely occurs in the setting of ADM and characterized by erythema that occurs directly over the ADM (16,17).
Ultrasonographic features of implanted ADM when used as supporting structure for implants are nonspecific and may include a heterogeneous yet predominantly hypoechoic mass with indistinct margins with or without nodularity corresponding to the palpable area of concern (4).
This manuscript is important as it highlights that this is a new procedure that is being offered at some centers following lumpectomy, and to date, there is very limited data on using ADM for volume replacement after BCS.
A recently published prospective study from South Korea enrolled 120 breast cancer patients with mean age (51.7) years undergoing BCS and volume replacement procedure with ADM. The authors of that study described using immediate diced ADM filling, and concluded the safety of this new approach. In that series, the overall complications rate was (20.5%) until 6 months follow-up and most frequently included seroma, hematoma, RBS and fat necrosis. Reoperation rate was (8.5%), and explantation of ADM was indicated in (1.7%) patients due to RBS and hematoma. The cosmetic and overall satisfaction questionnaires showed that more than 90% of the patients were strongly satisfied with the results of that reconstructive procedure (3).
Our patient and her primary surgeon did not report any short-term complications following the procedure.
There are no long-term follow up studies of this technique unfortunately and the impact of this technique on local recurrence rates is unknown. The additional cost and long-term cosmetic results have not been described.
We have no experience with this technique at our center (CCAD) and it is not one that is used widely in the United States. It is important to be aware that it is being performed at some centers globally. Further, there are few reports of the early breast imaging features of this technique. The case highlights the characteristic imaging features which can be useful to others who encounter patients who undergo similar procedures to facilitate their care. The pitfalls of this case report include providing care in multiple centers and using a rather new reconstructive procedure without detailed description to aid continuation between treating centers.
The diagnostic imaging challenges that can sometimes come with excluding a malignant process from fat necrosis are quite similar to that experienced in excluding malignancy from ADM. In similar cases, clinical correlation helps increase confidence in imaging diagnosis (4).
The overall prognosis of this case is good based on the early stage of her disease, adequate surgery, tumor biology and compliance to adjuvant treatment regimens.
Conclusions
This case describes the radiological features 6 months after using diced ADM in immediate reconstruction after BCS. These findings remain diagnostically challenging for radiologists and surgeons. Definitive diagnosis relies on clinical correlation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-93
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-93). SG serves as an unpaid editorial board member of Annals of Breast Surgery from Apr. 2020 to Mar. 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995;21:243-8. [Crossref] [PubMed]
- Sorkin M, Qi J, Kim HM, et al. Acellular dermal matrix in immediate expander/implant breast reconstruction: a multicenter assessment of risks and benefits. Plast Reconstr Surg 2017;140:1091-100. [Crossref] [PubMed]
- Gwak H, Jeon YW, Lim ST, et al. Volume replacement with diced acellular dermal matrix in oncoplastic breast-conserving surgery: a prospective single-center experience. World J Surg Oncol 2020;18:60. [Crossref] [PubMed]
- Lee CU, Bobr A, Torres-Mora J. Radiologic-Pathologic Correlation: Acellular Dermal Matrix (Alloderm®) Used in Breast Reconstructive Surgery. J Clin Imaging Sci 2017;7:13. [Crossref] [PubMed]
- Lee CU, Clapp AJ, Jacobson SR. Imaging features of AlloDerm(®) used in postmastectomy breast reconstructions. J Clin Imaging Sci 2014;4:19. [Crossref] [PubMed]
- Buck DW, Heyer K, Wayne JD, et al. Diagnostic dilemma: Acellular dermis mimicking a breast mass after immediate tissue expander breast reconstruction. Plast Reconstr Surg 2009;124:174e-6e. [Crossref] [PubMed]
- Parvizi D, Haas F, Peintinger F, et al. First experience using contrast-enhanced ultrasound to evaluate vascularisation of acellular dermal matrices after implant-based breast reconstruction. Breast J 2014;20:461-7. [Crossref] [PubMed]
- Seon Kim Y. Ultrasonography findings of AlloDerm® used in postmastectomy alloplastic breast reconstruction: A case report and literature review. Iran J Radiol 2016;13:e38252 [Crossref] [PubMed]
- Butler CE, Langstein HN, Kronowitz SJ. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg 2005;116:1263-75; discussion 1276-7. [Crossref] [PubMed]
- Chaplin JM, Costantino PD, Wolpoe ME, et al. Use of an acellular dermal allograft for dural replacement: an experimental study. Neurosurgery 1999;45:320-7. [Crossref] [PubMed]
- Russo AL, Taghian AG. Fat necrosis of the breast in the accelerated partial breast irradiation era: the need for a universal grading system. Breast Cancer Res Treat 2013;140:1-11. [Crossref] [PubMed]
- Paprottka FJ, Krezdorn N, Sorg H, et al. Evaluation of complication rates after breast surgery using acellular dermal matrix: median follow-up of three years. Plast Surg Int 2017;2017:1283735 [Crossref] [PubMed]
- Paprottka FJ, Krezdorn N, Sorg H, et al. Evaluation of complication rates after breast surgery using acellular dermal matrix: median follow-up of three years. Plast Surg Int 2017;2017:1283735 [Crossref] [PubMed]
- Ortiz JA. Clinical outcomes in breast reconstruction patients using a sterile acellular dermal matrix allograft. Aesthetic Plast Surg 2017;41:542-50. [Crossref] [PubMed]
- Lewis P, Jewell J, Mattison G, et al. Reducing postoperative infections and red breast syndrome in patients with acellular dermal matrixbased breast reconstruction: the relative roles of product sterility and lower body mass index. Ann Plast Surg 2015;74:S30-2. [Crossref] [PubMed]
- Ganske I, Hoyler M, Fox SE, et al. Delayed hypersensitivity reaction to acellular dermal matrix in breast reconstruction: the red breast syndrome? Ann Plast Surg 2014;73:S139-43. [Crossref] [PubMed]
- Wu PS, Winocour S, Jacobson SR. Red breast syndrome: a review of available literature. Aesthetic Plast Surg 2015;39:227-30. [Crossref] [PubMed]
Cite this article as: Iskanderian RR, Masri M, Nawaz N, Grobmyer SR. Acellular dermal matrix used for lumpectomy cavity volume replacement mimicking as breast cancer recurrence: a case report. Ann Breast Surg 2021;5:20.