Is it breast cancer? —common dermatologic disorders found on the breast
Approximately 10% of all outpatient visits in the United States are for dermatologic complaints (1). Most breast surgeons are comfortable identifying the asymptomatic, benign skin lesions, such as skin tags, nevi, and blackheads. Most other skin disorders are seen and managed by dermatologists (1). However, when these other skin disorders appear on the breast as the main or only site of involvement, both patients and physicians can become concerned about Paget’s disease, inflammatory breast cancer, or some other unusual manifestation of breast cancer. Fortunately, most skin diseases of the breast are just that, benign dermatologic diseases.
To make an accurate diagnosis, a thorough history and physical are needed. As with any other patient with breast concerns, family history, history of autoimmune diseases, other health history, medication list, immunization status and recent breast imaging can be helpful. Details regarding initial symptoms, changes in the skin lesions, duration, prior episodes and changes in medication or topical contacts should be obtained. A skin biopsy is often required for definitive diagnosis or at least to rule out malignancy (Table 1).
Table 1
| Condition | Breast location | Other common locations | Painful | Pruritic | Erythematous | Scaly | Risk factors | Punch biopsy useful |
|---|---|---|---|---|---|---|---|---|
| Inflammatory breast cancer | Whole breast | None | Yes | Maybe | Yes | No | Women, younger age, obesity, Blacks | Maybe |
| Paget’s disease | Unilateral nipple | None | No | No | Yes | Yes | Usual breast cancer risk factor | Yes |
| Herpes zoster | Linear thoracic dermatome | Anywhere | Yes | No | No | No | Immuno-compromised, women, Caucasians | No |
| Nipple thrush | Nipple | None | Yes | No | Yes | Yes | Breast feeding | No |
| Intertrigo | Inframammary fold, axilla | Groins, buttocks | No | No | Yes | No | Obesity, diabetes, HIV, large breast size | No |
| Allergic dermatitis | Any | Anywhere | No | Yes | Yes | No | – | No |
| Eczema | Bilateral nipple | Anywhere | No | Yes | Yes | Yes | Family history | Yes |
| Psoriasis | Anywhere | Anywhere | Yes | Yes | Yes | Yes | Family history, smoking | Yes |
| Inverse psoriasis | Inframammary fold | None | Yes | Yes | Yes | Yes | Family history, smoking | Yes |
| Reynaud’s | Nipple | Hands | Yes | No | Bi or triphasic color change | No | Known disease in hands | No |
| Hidradenitis suppurativa | Inframammary fold, axilla | Groins, buttocks, perineum, genitals | Yes | No | No | No | Possibly smoking, obesity, family history | No |
| Morphea | Any | Any | No | No | Yes | No | Radiation, women | Yes |
| Calciphylaxis | Any | Abdomen, buttocks, thighs, flanks | Yes | No | No | No | End stage renal disease, obesity, diabetes, women, hyperphosphatemia, warfarin | No |
The breast is composed of layers—skin, pre-mammary fat, mammary layer, and retromammary fat, lying on top of muscle and chest wall. The nipple and areolar complex (NAC) is specialized skin which includes smooth muscle, Montgomery glands which provide lubrication during lactation and double the melanin count of the surrounding skin (2-4). The types of skin conditions seen on the skin of the breast can be categorized as malignant, infectious, or inflammatory. Many of these conditions can develop or exacerbate during breastfeeding.
Malignant
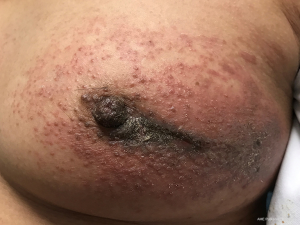
For comparison with benign entities affecting the skin of the breast, there are several malignancies that can present as changes in the skin of the breast. Primary skin malignancies such melanoma, Bowen’s disease and basal cell carcinoma can be seen on the skin of the breast, although rare. Basal cell carcinoma is seen more commonly in men given the higher sun exposure of the chest in men (5). The most common malignancy involving the skin of the breast is breast cancer. Breast cancer presents with skin involvement in 6–10% of cases (6-8). This can be either by local skin involvement from the underlying cancer or as a true inflammatory breast cancer due to tumor invasion of dermal lymphatics. National Comprehensive Cancer Network (NCCN) guidelines describe the inflammatory breast cancer presentation as painful, erythematous, and edematous breast skin over at least one-third of the breast. These patients will often present with axillary adenopathy as well. If a skin punch biopsy reveals carcinoma involving the dermal lymphatics, it can help make the diagnosis; however, a negative punch biopsy does not exclude a diagnosis of inflammatory breast cancer (9,10). Direct skin involvement by local extension can present with focal edema, erythema, ulceration or skin nodules while subtle skin involvement can present as skin retraction, nipple inversion or other changes related to involvement of the underlying Cooper’s ligaments (9). A more unusual breast cancer presentation is Paget’s disease of the breast which presents with thickened, eczematous skin on the NAC, sometimes also with pigment changes and overlying crust (Figure 1). Paget’s represents 1% of all breast cancers and the majority involve the breast parenchyma in addition to the skin (11,12). Physical examination, skin biopsy and breast imaging will often distinguish breast cancer involving the skin from other primary skin disorders.
Infectious
Outside of puerperal and non-puerperal mastitis, there are other infectious entities that can be found involving the skin of the breast, such as herpes zoster and nipple thrush. Herpes zoster (shingles) is caused by a reactivation of the varicella zoster virus as immunity wanes due to age or immunosuppression. Approximately, 30% of adults will develop herpes zoster over their lifetime. Women are at increased risk while blacks are at reduced risk for herpes zoster (13,14). Initial presentation is often abnormal skin sensations such as burning, stinging, or tingling, followed by vesicular skin lesions 2–3 days later along a dermatome (Figure 2) (15). Herpes zoster can be seen along any dermatome but the thoracic dermatomes along the intercostal nerves are commonly involved and therefore, can involve the skin of the breast. Antivirals such as acyclovir, valacyclovir, or famciclovir can be used to shorten the course while oral steroids can be used in conjunction with these antivirals to reduce pain and promote early healing (15). In 2017, a recombinant varicella zoster virus vaccine was approved. The vaccine reduced the incidence of herpes zoster by 96% in those receiving the vaccine. The vaccine is approved for adults 50 years and older for prevention of herpes zoster (16).
Nipple thrush presents typically with burning, shooting, or stabbing nipple pain radiating to the chest wall in a breastfeeding mother. Clinical findings are often less impressive than the patient’s symptoms. Candida albicans is typically the causative organism. Skin of the NAC may be erythematous, shiny, and flaky (17). Infants may have a fiery red diaper rash or white patches on the oral mucosa. Both the mother and infant must be treated to prevent reinfection. Gentian violet is considered safe and effective for both mother and infant. Concentrated solution less than 0.5% can be applied once daily for no more than 7 days to the infant’s mouth and then immediately breastfeed. Infant’s mouth and mother’s nipple will be purple after feeding. If mother’s nipple is not purple at the end of the feed, then the gentian violet should be applied directly to the nipple (18,19). Alternatively, 1ml nystatin suspension can be applied to the infant’s mouth after each feed for 7–10 days while topical nystatin cream can be applied to the NAC twice daily for 14 days (18). If topical treatment was unsuccessful, oral fluconazole can be used both for the mother as well as infants over 6 months of age (19). Also, all linens and bras should be washed in hot water with distilled vinegar as well as washing all pacifiers, nipples, breast pump shields and any other parts that contact breast milk (18).
Inflammatory
There are a number of inflammatory skin conditions which can be seen on the breast. Friction and moisture occurring in skin folds can cause areas of maceration and erythema known as intertrigo. Inframammary folds, axillae, interdigital and intergluteal folds are commonly affected. Known risk factors include obesity, diabetes, large pendulous breasts, and HIV (20). Treatment involves use of barrier creams and absorbent products to minimize friction and manage moisture. Short course of low potency steroid cream can be used for symptom relief. If no improvement is achieved with above measures, an alternate diagnosis such as inverse psoriasis should be considered (20,21). Bacterial and fungal superinfection can occur with intertrigo. The most common organism is candida. This will typically present with satellite pustules and papules. Topical antifungal cream (nystatin or azole) with barrier creams should be used for initial treatment (20,21). Alternatively, a combination steroid cream with azole can be used but is often cost-prohibitive and can cause atrophy of the skin if overused. Oral fluconazole can be used when topical treatments fail (22).
Allergic contact dermatitis is the most common dermatologic diagnosis identified by internists, family physicians and pediatricians (1). History should include asking about new soaps, laundry detergent, fabric softeners, personal hygiene products, and bras. Other common contact allergens are also tattoos, piercings and nail polish (23). In breastfeeding mothers, ingredients in nipple creams such as lanolin, chamomile, aloe vera, chlorhexidine, topical vitamin E, fragrances, tea bags as well as solid foods in the infant’s diet can all be associated with allergic contact dermatitis (24). Low to moderate potency topical steroids, oral steroids 4 h prior to lactation and photosynthesis are all options for treatment while breastfeeding (25-27). Topical steroids should be wiped from the NAC prior to feeding to minimize infant exposure. Expressed breast milk can be used to wipe off topical steroids as it is less irritating than water or other materials (24). Another common contact allergen on the breast that a breast surgeon is likely to encounter is 2-octylcyanoacrylate glue used for skin closure. This is commonly found branded as Dermabond (Ethicon, Inc., Somerville, NJ, United States) or LiquiBand (Advanced Medical Solutions Ltd., Winsford, Cheshire, United Kingdom). Breakdown products of these glues include formaldehyde and cyanoacetate which can induce a reaction, especially in open wounds or areas of thin skin (28,29). Erythema, urticaria, and/or pruritis starting at and then spreading from the incision without fever or tenderness is the typical presentation (Figure 3). Most of these reactions were seen within 5–7 days of surgery (30,31). In a single series of 102 consecutive patients after breast surgery, 14% had contact dermatitis to Dermabond confirmed by scratch test; all these patients also reacted to LiquiBand and therefore the reaction was not brand specific (30). Contact dermatitis has also been reported with the use of Dermabond Prineo (Ethicon, Inc., Somerville, NJ, United States) which is 2-octylcyanoacetate combined with a self-adhesive polyester mesh (32). Treatment includes removing the glue and adding topical steroid cream, either over the counter 1% or prescription 2.5%, depending on severity. For cases that do not improve within a few days, steroid dose pack can be added (30-32). Contact dermatitis of the breast has also been reported with a bra with a gel insert containing propylene glycol (33), a bra containing P-tert-butylphenol formaldehyde resin on the inner bra fabric (34) and clothing dye disperse blue 106 (35).
Eczema is the most common presentation of atopic dermatitis of the breast and usually involves the NAC (5). Presentation may include erythema, scale formation, weeping, crusting, fissuring, erosion, excoriation or lichenification of the NAC skin (36,37). Eczema usually involves the NAC bilaterally (Figure 4). This can be seen in women with a history of eczema elsewhere on the body. Initial presentation can also be precipitated by breastfeeding due to the feeding action or contact with food residue in the infant’s mouth (37). Management includes avoiding known irritants, use of topical steroids, lubricants, and antibiotics (5).
Psoriasis can present on the skin of the breast and NAC. On the NAC, this will present as a well-demarcated, erythematous plaque with fine, micaceous scales. Nursing mothers with a history of psoriasis may develop exacerbations due to the irritation from latch on and suckling. When presenting in the inframammary fold, it is known as inverse (or intertriginous) psoriasis and can be mistaken for other dermatoses as it will lack scale and maceration and is often well-demarcated. First line therapy involves 2- to 4-week course of low potency topical steroids, topical immunomodulators and calcitriol and calcipotriene (topical vitamin D) (38). Topical immunomodulators are contraindicated in breastfeeding due to potential oral absorption by the infant. Calcipotriene, however, can be used during breastfeeding as long as less than 20% body surface area is covered daily (18). As a systemic therapy, phototherapy with ultraviolet B, methotrexate, and biologic agents such as etanercept, adalimumab, infliximab, alefacept, and ustekinumab can be used. Data is limited on safety of biologic agents during breastfeeding (19)
Raynaud’s phenomenon of the nipple is caused by vasospasm of the arterioles causing intermittent ischemia. Biphasic or triphasic color change of the nipple can be seen (39). History of Raynaud’s of the hands can be seen in 20% of women of childbearing age and is a risk factor for Raynaud’s phenomenon of the nipple (40). This phenomenon has also been correlated with a history of breast surgery (39). This diagnosis should be considered in a breastfeeding mother who has had at least 4 weeks of nipple pain in spite of treatment for bacterial and fungal causes of nipple pain. Treatment should include avoiding cold temperatures, wearing warm clothes, taking hot showers twice daily prior to breastfeeding, and avoiding caffeine, nicotine, and any other vasoconstrictive drugs (39). Nifedipine 30–60 mg/day sustained release is approved by the American Academy of Pediatrics for breastfeeding mothers. Most will improve substantially with 14 days of treatment (41). If adverse effects such as dizziness, headache, hypotension, nausea, or tachycardia are noted, a decrease in dose to 10 mg/day may still be effective and better tolerated (39) For medication-averse patients, high-dose vitamin B6 at 100 mg twice daily can be used based on anecdotal evidence (24).
Hidradenitis suppurativa (HS) is a chronic inflammatory condition affecting the hair follicles in the gland-bearing regions including armpits, inframammary area of the breasts, buttocks, genital areas, groins, and perianal area (42). The inflammation of these apocrine glands leads to boils, abscess, sinus tracts, malodorous drainage and ultimately scarring. Patients with HS report low quality of life (43,44), general health (45) and depression (46). HS can be seen in 1–4% of the population (42,47,48). Onset is typically seen after puberty, and peak incidence is in the 20s–30s (49). Incidence is more common in women by a 3:1 ratio (47,48,50). Women more commonly affected on the breasts and inguinal regions while men are more likely to have involvement of the buttocks (42,51). However, there does not appear to be a sex-related difference in severity (51). Severity is graded using the Hurley severity scale (52). Development of HS has been associated with smoking (43,53), increased body mass index (BMI) (43,48), and family history of HS (54,55). Other studies have not substantiated these associations (50,56). Increased disease severity has been associated with increased BMI, atypical location of HS, absence of family history, history of severe acne, smoking and obesity (43,50,51). Medical management is preferable if possible. Medical treatments include topical erythromycin or clindamycin, oral clindamycin, dapsone, tetracycline, or minocycline (57-59). Several years ago, adalimumab was approved for moderate to severe HS. Two multicenter trials demonstrated ≥50% reduction in abscess and nodule count with no new abscesses or draining fistulas after 12 weeks of treatment (60). Other medical treatments include oral zinc as well as topical or oral retinoids. For medical failures, wide local excision, CO2 laser ablation, radiotherapy and radiofrequency treatment are also options. (59,61-63).
Morphea, also known a localized scleroderma, is rare with less than 3 cases per 100,000 in the United States (64). Histologically, there is increased collagen with nonspecific inflammatory changes in the dermis and atrophy of the epidermis (65). The etiology is unknown but thought to be an autoimmune disease given that antibodies to type IV collagen have been identified in scleroderma (66,67). It is most common in women. There have been case reports of large plaque morphea being associated with lung, hematologic and breast cancer, prompting consideration of this as a possible paraneoplastic syndrome (64). Morphea can also be a consequence of radiation. Radiation-induced morphea occurs in 2 of 1,000 cases and presents as a gradually hardened, reddened, uncomfortable skin corresponding to the radiation field (68) (Figures 5,6). It has been suggested that the development of radiation-induced morphea may be a result of release of radiation-induced neoantigens affecting fibroblasts, endothelial proteins, collagen, or elastin proteins (69). Steroids, methotrexate, and UVA-1 phototherapy can be used to slow progression and manage symptoms (68).
Calciphylaxis is a multifactorial cutaneous, vascular disease known also as calcific uremic arteriolopathy and uremic small artery disease. It is characterized by painful, non-healing wounds which occur most commonly in patients with end-stage renal disease (70). The skin lesions develop after medial calcification, intimal fibrosis of arterioles and thrombotic occlusion. Early presentation can mimic breast cancer (71). Calciphylaxis lesions start as indurated, tender plaques which progress to stellate, necrotic plaques and ultimately cutaneous ulceration. These typically involve the adipose rich portions of the body, including the abdomen, breasts, buttocks, flanks, thighs, and trunk (72). The incidence is 1–4% of patients on dialysis. Risk factors include female sex, obesity, diabetes, hyperphosphatemia, chronic/end-stage renal disease, and warfarin use (73-76). The prognosis for patients with calciphylaxis is poor with 2-year survival rates as low as 20% while the mortality rate is greater than 80% in patients who have developed cutaneous ulcerations (77). Multidisciplinary approach including specialists in dermatology, nephrology, wound care, and pain management is important. Diagnosis can by confirmed with small (3–5 mm) punch biopsy at the edge of a lesion, but this could also worsen the ulcer. Early consultation with a pain management specialist or depending on patient’s overall health status, palliative care specialist, is warranted as pain is the most significant complaint. Surgical debridement is controversial, but the primary mortality cause is sepsis; therefore, debridement of infected lesions is indicated. Maggot debridement, chemical debridement, negative pressure dressings, hyperbaric oxygen and skin grafting are other wound care options that can be utilized (69). Sodium thiosulfate (STS) has become one of the mainstays for treatment of calciphylaxis. STS can chelate calcium salts from the skin, subcutaneous tissue, and organs. Effective dosages have been reported from 5 to 25 mg three times per week during or after hemodialysis, providing improvement in pain and ulcers in 70% of patients (78-84). Correction of calcium and phosphate abnormalities is another key in treatment. This can include parathyroidectomy for patients with concomitant hyperparathyroidism but is often precluded by the surgical risk of these patients (70).
In closing, a number of benign skin entities can present on the breast. A complete history and physical exam are warranted as well as breast imaging to exclude breast cancer. Skin punch biopsy is often useful in eliminating malignancy as the etiology for the skin changes. Many of the inflammatory conditions can be treated initially with low potency topical steroids. Early involvement by a dermatologist can be useful.
Acknowledgments
The author would like to thank the staff of the Bronstein Library at Baptist Memorial Hospital-Memphis for their assistance with the literature search, Drs. Thomas Throckmorton and Jennifer Zakhireh for their proofreading and editorial assistance, and Drs. Christine Dauphine and Shawna Willey for their assistance with the figures.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Katharine Yao) for the series “A Practical Guide to Management of Benign Breast Disease” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-97). The series “A Practical Guide to Management of Benign Breast Disease” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fleischer AB, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care 2000;6:1149-56. [PubMed]
- Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia 2000;5:119-37. [Crossref] [PubMed]
- Dean N, Haynes J, Brennan J, et al. Nipple-areolar pigmentation: histology and potential for reconstitution in breast reconstruction. Br J Plast Surg 2005;58:202-8. [Crossref] [PubMed]
- Sarhadi NS, Shaw-Dunn J, Soutar DS. Nerve supply of the breast with special reference to the nipple and areola: Sir Astley Cooper revisited. Clin Anat 1997;10:283-8. [Crossref] [PubMed]
- Whitaker-Worth DL, Carlone V, Susser WS, et al. Dermatologic diseases of the breast and nipple. J Am Acad Dermatol 2000;43:733-51; quiz 752-4. [Crossref] [PubMed]
- Dossus L, Benusiglio P. Lobular breast cancer: incidence and genetic and non-genetic risk factors. Breast Cancer Res 2015;17:37-45. [Crossref] [PubMed]
- Güth U, Moch H, Herberich L, et al. Noninflammatory breast carcinoma with skin involvement. Cancer 2004;100:470-8. [Crossref] [PubMed]
- Güth U, Wight E, Schotzau A, et al. A new approach in breast cancer with non-inflammatory skin involvement. Acta Oncol 2006;45:576-83. [Crossref] [PubMed]
- NCCN guidelines. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 1/17/2021.
- Robertson FM, Bondy M, Yang W, et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin 2010;60:351-75. Erratum in: CA Cancer J Clin 2011 Mar-Apr;61(2):134. Ueno, Naoto [corrected to Ueno, Naoto T]. [Crossref] [PubMed]
- Güth U, Singer G, Schotzau A, et al. Scope and significance of non-uniform classification practices in breast cancer with non-inflammatory skin involvement: a clinicopathologic study and an international survey. Ann Oncol 2005;16:1618-23. [Crossref] [PubMed]
- Berg JW, Hutter R. Breast cancer. Cancer 1995;75:257-69. [Crossref] [PubMed]
- Johnson BH, Palmer L, Gatwood J, et al. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis 2015;15:502. [Crossref] [PubMed]
- Cohen JI. Clinical practice: Herpes zoster. N Engl J Med 2013;369:255-63. [Crossref] [PubMed]
- Saguil A, Kane S, Mercado M, et al. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician 2017;96:656-63. [PubMed]
- Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087-96. [Crossref] [PubMed]
- Morrill JF, Heinig MJ, Pappagianis D, et al. Risk factors for mammary candidosis among lactating women. J Obstet Gynecol Neonatal Nurs 2005;34:37-45. [Crossref] [PubMed]
- Barrett ME, Heller MM, Fullerton Stone H, et al. Dermatoses of the breast in lactation. Dermatol Ther 2013;26:331-6. [Crossref] [PubMed]
- Hale T. Medications and mothers’ milk. 14th edition. Amarillo, TX: Hale Pub., L.P., 2010.
- Janniger CK, Schwartz RA, Szepietowski JC, et al. Intertrigo and common secondary skin infections. Am Fam Physician 2005;72:833-8. [PubMed]
- Valenti L. Topical treatment of intertriginous candida infections. Mycoses 2008;51:44-5. [Crossref] [PubMed]
- Coldiron BM, Manders S. Persistent candida intertrigo treated with fluconazole. Arch Dermatol 1991;127:165-6. [Crossref] [PubMed]
- Kapur N, Goldsmith P. Nipple dermatitis – not all what it ‘seams’. Contact Dermatitis 2001;45:44-5. [Crossref] [PubMed]
- Waldman RA, Finch J, Grant-Kiels JM, et al. Skin diseases of the breast and nipple: Inflammatory and infectious diseases. J Am Acad Dermatol 2019;80:1483-94. [Crossref] [PubMed]
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: Part II. Lactation. J Am Acad Dermatol 2014;70:417.e1-10; quiz 427. [Crossref] [PubMed]
- Kurizky PS, Ferreira C, Nogueria L, et al. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol 2015;90:367-75. [Crossref] [PubMed]
- Gaidos J, Kane S. Biologics in pregnancy and breastfeeding. In: Cheifetz AS, Feuerstein JD. editors. Treatment of Inflammatory Bowel Disease with Biologics. Cham, Switzerland: Springer, 2018:81-99.
- Caton AM, Dauphine C. Allergic contact dermatitis after repeated exposure to dermabondTM. Am Surg 2014;80:520-2. [Crossref] [PubMed]
- Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octylcyanoacrylate. Arch Dermatol 2008;144:814-5. [Crossref] [PubMed]
- Nigro LC, Parkerson J, Nunley J, et al. Should we stick with surgical glues? The incidence of dermatitis after 2-octylcyanoacetate exposure in 102 consecutive breast cases. Plast Reconstr Surg 2020;145:32-7. [Crossref] [PubMed]
- Howard BK, Downey SE. Contact dermatitis from Dermabond. Plast Reconstr Surg 2010;125:252e-253e. [Crossref] [PubMed]
- Davis MDP, Stewart M. Severe allergic contact dermatitis to Dermabond Prineo, a topical skin adhesive of 2-octylcyanoacetate increasingly used in surgeries to close wounds. Dermatitis 2016;27:75-6. [Crossref] [PubMed]
- Lamb SR, Ardley HC, Wilkinson SM. Contact allergy to propylene glycol in brassiere padding inserts. Contact Dermatitis 2003;48:224-5. [Crossref] [PubMed]
- Herro EM, Freidlander SF, Jacob SE. Bra-associated allergic contact dermatitis: p-tert-butylphenol formaldehyde resin as the culprit. Pediatr Dermatol 2012;29:540-1. [Crossref] [PubMed]
- Dawes-Higgs E, Freeman S. Allergic contact dermatitis caused by the clothing dye, disperse blue 106, an important contact allergen that may be frequently missed. Australas J Dermatol 2004;45:64-6. [Crossref] [PubMed]
- Ward KA, Burton JL. Dermatologic diseases of the breast in young women. Clin Dermatol 1997;15:45-52. [Crossref] [PubMed]
- Amir L. Eczema of the nipple and breast: a case report. J Hum Lact 1993;9:173-5. [Crossref] [PubMed]
- Khosravi H, Siegel MP, Van Voorhees AS, et al. Treatment of Inverse/Intertriginous Psoriasis: Updated Guidelines from the Medical Board of the National Psoriasis Foundation. J Drugs Dermatol 2017;16:760-6. [PubMed]
- Barrett ME, Heller MM, Fullerton Stone H, et al. Raynaud phenomenon of the nipple in breastfeeding mothers: An underdiagnosed cause of nipple pain. JAMA Dermatol 2013;149:300-6. [Crossref] [PubMed]
- Anderson JE, Held N, Wright K. Raynaud’s phenomenon of the nipple: a treatable cause of painful breastfeeding. Pediatrics 2004;113:e360-4. [Crossref] [PubMed]
- American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics 2001;108:776-89. [Crossref] [PubMed]
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J 2014;90:216-21. [Crossref] [PubMed]
- Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacoo smoking and obesity. Br J Dermatol 2009;161:831-9. [Crossref] [PubMed]
- Jemec GB, Heidenheim M, Nielsen NH. Hidradenitis suppurativa: characteristics and consequences. Clin Exp Dermatol 1996;21:419-23. [Crossref] [PubMed]
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of Life Group of the French Society of Dermatology. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol 2007;56:621-3. [Crossref] [PubMed]
- Matusiak L. Bieniek, Szepietowski JC. Psychological aspects of hidradenitis suppurativa. Acta Derm Venereol 2010;90:264-8. [Crossref] [PubMed]
- Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996;35:191-4. [Crossref] [PubMed]
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol 2008;59:596-601. [Crossref] [PubMed]
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2000;14:389-92. [Crossref] [PubMed]
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol 2009;61:51-7. [Crossref] [PubMed]
- Bianchi L, Caposiena Caro RD, Ganzetti G, et al. Sex-related differences of clinical features in hidradenitis suppurativa: analysis of an Italian-based cohort. Clin Exp Dermatol 2019;44:e177-80. [Crossref] [PubMed]
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH Jr. editors. Dermatologic surgery: principles and practice. 2nd edition. New York: Marcel Dekker, 1996:623-45.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology 1999;198:261-4. [Crossref] [PubMed]
- Von Der Werth JM, Williams HC, Raeburn JA. The clinical genetics of hidradenitis suppurativa revisited. Br J Dermatol 2000;142:947-53. [Crossref] [PubMed]
- Al-Ali FM, Ratnamala U, Mehta TY, et al. Hidradenitis suppurativa (or acne inversa) with autosomal dominant inheritance is not linked to chromosome 1p21.1-1q25.3 region. Exp Dermatol 2010;19:851-3. [Crossref] [PubMed]
- Matusiak L, Bieniek A, Szepietowski JC. Hidradenitis suppurativa and associated factors: still unsolved problems. J Am Acad Dermatol 2009;61:362-5. [Crossref] [PubMed]
- Jemec GB. Hidradenitis suppurativa. N Engl J Med 2012;366:158-64. [Crossref] [PubMed]
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619-44. [Crossref] [PubMed]
- Rambhatla PV, Lim H, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol 2012;148:439-46. [Crossref] [PubMed]
- Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med 2016;375:422-34. [Crossref] [PubMed]
- Li EN, Mofid M, Goldberg N, et al. Surgical management of hidradenitis suppurativa of the nipple-areolar complex. Ann Plast Surg 2004;52:220-3. [Crossref] [PubMed]
- Lapins J, Sartorius K, Emtestam L. Scanner-assisted carbon dioxide laser surgery: a retrospective follow-up study of patients with hidradenitis suppurativa. J Am Acad Dermatol 2002;47:280-5. [Crossref] [PubMed]
- Mahmoud BH, Tierney E, Hexsel C, et al. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodynmium: yttrium-aluminum-garnet laser. J Am Acad Dermatol 2010;62:637-45. [Crossref] [PubMed]
- Desmond BL, Blattner CM, Young Iii J. Generalized morphea as the first sign of breast carcinoma: a case report. Dermatol Online J 2016;22:13030/qt2tr4496q.
- Chiriac A, Podoleanu C, Coros M, et al. Localized morphea developing in a scar after breast carcinoma surgery in the absence of radiotherapy. J Cutan Med Surg 2016;20:606. [Crossref] [PubMed]
- Young EM Jr, Barr RJ. Sclerosing dermatoses. J Cutan Pathol 1985;12:426-41. [Crossref] [PubMed]
- Mackel AM, DeLustro F, Harper FE, et al. Antibodies to collagen in scleroderma. Arthritis Rheum 1982;25:522-31. [Crossref] [PubMed]
- Lim D, Johnston S, Novakovic L, et al. Radiation-induced morphea treated with UVA-1 phototherapy. Clin Exp Derm 2014;39:612-5. [Crossref] [PubMed]
- Schaffer JV, Carroll C, Dvoretsky I, et al. Postirradiation morphea of the breast. Presentation of two cases and review of the literature. Dermatology 2000;200:67-71. [Crossref] [PubMed]
- García-Lozano JA, Ocampo-Candiani J, Martinez-Cabriales SA, et al. An update on calciphylaxis. Am J Clin Dermatol 2018;19:599-608. [Crossref] [PubMed]
- Hall DJ, Gentile LF, Duckworth LV, et al. Calciphylaxis of the breast: a case report and literature review. Breast J 2016;22:568-72. [Crossref] [PubMed]
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci 2016;351:217-27. [Crossref] [PubMed]
- Mazhar AR, Johnson RJ, Gillen D, et al. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int 2001;60:324-32. [Crossref] [PubMed]
- Tian F, Patterson AT, Davick JJ, et al. The cutaneous expression of vitamin K-dependent and other osteogenic proteins in calciphylaxis stratified by clinical features and warfarin use: a case control study. J Am Acad Dermatol 2016;75:840-842.e1. [Crossref] [PubMed]
- Dobry AS, Ko LN, St John J, et al. Association between hypercoagulable conditions and calciphylaxis in patients with renal disease: a case-control study. JAMA Dermatol 2018;154:182-7. [Crossref] [PubMed]
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol 2016;27:3421-9. [Crossref] [PubMed]
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007;56:569-79. [Crossref] [PubMed]
- Vedvyas C, Winterfeld LS, Vleugels RA. Calciphylaxis: a systematic review of existing and emerging therapies. J Am Acad Dermatol 2012;67:e253-60. [Crossref] [PubMed]
- Peng T, Zhuo L, Wang Y, et al. A systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology 2018;23:669-75. [Crossref] [PubMed]
- Bourgeois P, De Haes P. Sodium thiosulfate as a treatment for calciphylaxis: a case series. J Dermatolog Treat 2016;27:520-4. [Crossref] [PubMed]
- Noureddine L, Landis M, Patel N, et al. Efficacy of sodium thiosulfate for the treatment of calciphylaxis. Clin Nephrol 2011;75:485-90. [Crossref] [PubMed]
- Singh RP, Derendorf H, Ross EA. Simulation-based sodium thiosulfate dosing strategies for the treatment of calciphylaxis. Clin J Am Soc Nephrol 2011;6:1155-9. [Crossref] [PubMed]
- Farese S, Stauffer E, Kalicki R, et al. Sodium thiosulfate pharmacokinetics in hemodialysis patients and healthy volunteers. Clin J Am Soc Nephrol 2011;6:1447-55. [Crossref] [PubMed]
- Auriemma M, Carbone A, Di Liberato L, et al. Treatment of cutaneous calciphylaxis with sodium thiosulfate: two case reports and a review of the literature. Am J Clin Dermatol 2011;12:339-46. [Crossref] [PubMed]
Cite this article as: Throckmorton AD. Is it breast cancer? —common dermatologic disorders found on the breast. Ann Breast Surg 2021;5:28.