
Evolution in breast reconstruction using the omentum
Introduction
Breast reconstruction after mastectomy is increasingly being performed for breast cancer and genetically-susceptible patients. The primary approaches for breast reconstruction are implant-based or from autologous donor tissue. While implant-based reconstruction is more common, women are increasingly interested in autologous reconstruction techniques especially with the concerns about breast implant associated anaplastic large cell lymphoma (BIA-ALCL). The typical autologous donor sites are the abdomen, thigh, or gluteus; however, women who are thin or would prefer to minimize the morbidity associated with these donor sites have accelerated the search for an alternative autologous reconstructive option. One possibility that has yet to be frequently used for breast reconstruction is the omentum.
The omentum was first sought as a tool for breast reconstruction over 50 years ago. Kiricuta appears to be one of the first surgeons to have successfully used pedicled omental flaps to reconstruct the breast in Romania (1). Since then, the omentum has been used as a pedicled and free flap for autologous breast reconstruction, but is not considered a routine treatment option. A major challenge to the use of omentum is that it is a flat, unstructured sheet. Herein, we describe the evolution of using the omentum in reconstruction and how it is now a viable option for autologous breast reconstruction after mastectomy.
Methods
A literature review was performed on PubMed using the keywords of omentum, omental, breast, and reconstruction. The included manuscripts were studies describing surgical technique in human subjects and written in the English language. The titles and abstracts were manually reviewed. Manuscripts discussing the use of the omentum for non-breast reconstruction or where their complete text was not available were excluded.
Anatomic description
The greater omentum is typically the first structure encountered when entering the abdomen, covering the intra-abdominal organs. It arises from the greater curvature of the stomach, sometimes reaching the pelvis, and then folds on itself to enclose the transverse colon creating the gastrocolic ligament (2). On the right side, the attachments between the lesser curvature of the stomach and proximal duodenum to the liver create the gastrohepatic ligament, which is a part of the lesser omentum (2). On the left side, the greater omentum can extend from the fundus to the spleen forming the gastrosplenic ligament (3).
Its surface area varies from 300–1,500 cm2 and dimensions range between 14–36 cm in length to 20–46 cm in width (2,3). The weight of the omentum has been reported to be from 300–2,000 grams (2). It is composed of fatty tissue, arteries, veins, lymphatics, and lymph nodes. The primary blood supply originates from the celiac trunk, which gives off the right and left gastroepiploic (gastro-omental) vessels. The right gastroepiploic artery arises from the gastroduodenal artery while the left gastroepiploic artery arises from the splenic artery. Both arteries run between the layers of the greater omentum giving off gastric and omental branches, and eventually anastomose with one another. The gastroepiploic vessels are about a fingerbreadth from the greater curvature except where it is in close proximity to the pylorus. The right gastroepiploic artery has a larger diameter (2.8 mm) compared to the left gastroepiploic artery (2.2 mm) (3). The venous system of the omentum parallels the arteries and drains into the portal system (3).
Prior and current uses of the omentum
Historically, the omentum has been known as the ‘policeman of the abdomen’ with biological functions in immune-regulation, neovascularization, hemostasis, and tissue regeneration (2,3). It is a richly vascularized tissue that can easily replicate the shape of irregular defects and is useful for infected or irradiated wounds. The first reported use was to decrease the risk of bowel complications by creating adhesions between the bowel and the omentum in the early 1800s (3). Since then, it has been used in a variety of specialties including neurosurgery, gynecology, colorectal, cardiothoracic, orthopedics, and plastic and reconstructive surgery.
Pedicled omental flaps have been more commonly used compared to free flaps as a pedicled flap can reach distant areas without the added complexity of a microvascular anastomosis. They are extensively used for coverage of tissue defects, treatment of infections, or to supplement suture lines such as a bronchial fistula or after pulmonary resection, sternal infections, or perineal closures after APR, respectively (4,5). A retrospective review over 25 years reported that the most common use of an omental flap by plastic surgeons was for the sternum and orbit/face followed by the chest wall and scalp (6). The more common use of the omentum in breast cancer patients has been for chest wall coverage in women with fungating masses (7-9).
Breast reconstruction using the omentum
As breast reconstruction has an increasing role after mastectomy, the use of the omentum has been reported but remains an uncommon reconstructive option. It has been predominantly used as a pedicled flap for partial or total autologous breast reconstruction (10-16). Use as a free omental flap is rarely done after total mastectomy with increased applications in patients undergoing breast conserving therapy (10,17-20). Because it can be difficult to predict the size of the omentum, its use has most commonly been used for small- to medium-sized breasts with the possible need to supplement a small-sized omentum with a latissimus dorsi flap or breast implant (15,18,21).
While the most common autologous option for breast reconstruction is abdominal tissue, not all women qualify or desire this approach. Women who have had a prior abdominoplasty, limited abdominal wall soft tissue, or prefer to minimize their risk of abdominal wall morbidity are ideal candidates for an autologous omental free flap. A major challenge to using the omentum in the past was the morbidity associated with a laparotomy. Since the early 2000s, however, laparoscopy has been more widely accepted for harvesting the omentum and is now the standard approach (22,23).
The current reservations in using the omentum is the difficulty in predicting its volume pre-operatively (10,17). Various imaging modalities (ultrasonography, computed-tomography, magnetic resonance imaging) have been inaccurate in estimating the volume of the omentum, and patient weight or body mass index is not predictive (3). A group from Japan reviewed 200 patient cases who underwent oncoplastic surgery with an omental flap for reconstruction (21). More than one-third of their patients who had a nipple- or skin-sparing mastectomy had volume insufficiency in using the omentum alone necessitating the use of the latissimus dorsi or a tissue expander.
For these reasons, the omental fat-augmented free flap (O-FAFF) provides a viable alternative option for autologous breast reconstruction in patients. We recently reported the outcomes for 3 patients who had undergone this approach (24). This technique resulted in a volume comparable to the patients’ resected mastectomy volumes, as well as restoration of a natural breast shape. The viability of grafted fat was also confirmed using MRI where fat necrosis was not apparent. The final appearance of the reconstructed breast resembled an implant due to the projection afforded by O-FAFF can achieve with the surrounding ADM construct, but also maintained a soft consistency due to the presence of autologous tissue (24). The use of the thoracodorsal recipient vessels can result in a bridge deformity in the axilla. Therefore, the internal mammary vessels represent a more suitable recipient site (10,11,18,23).
Surgical technique
The omentum can be harvested through an open abdominal or minimally-invasive approach. Adhesiolysis is done if needed. It is then initially dissected off of the transverse colon and the short gastric vessels are divided. If attachments are present between the omentum and the anterior surface of the pancreas, these are divided as well to open the lesser sac (3). When a laparoscopic approach is used to harvest the omentum, the morbidity associated with the donor site decreases when compared to a laparotomy which can be complicated by hernias and delayed wound healing (10,22).
Depending on the use of the omentum as a pedicled or free flap, will determine the extent of omental dissection. If a pedicled omentum is needed for a local defect, the site of the vascular pedicle is decided. The gastroepiploic vessels on the opposite side are divided with division of the omentum from its attachments to the stomach. The omentum may need to be lengthened depending on the defect site it is being used for. One can better visualize the vascular arcade by shining a light through the omentum to determine the branches to be divided while preserving adequate blood supply and achieving length while minimizing tension on the pedicle. It is recommended that at the time of division for one or both of the gastroepiploic vessels, a large plastic clip be used to easily identify the vascular pedicle.
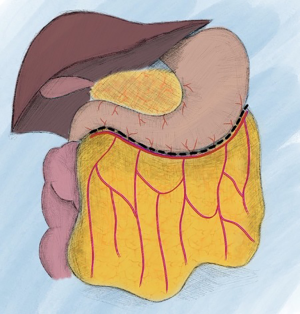
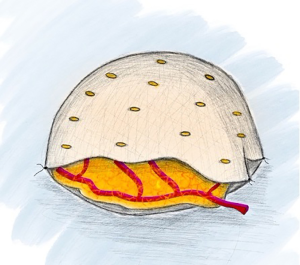
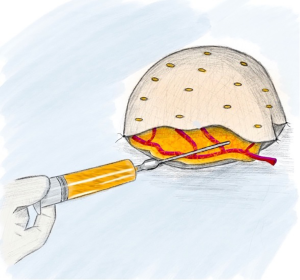
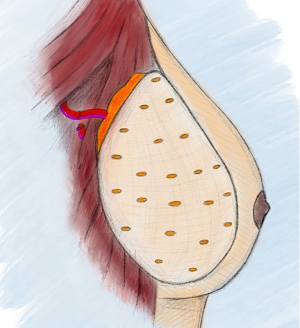
If a free omental flap for total breast reconstruction is to be performed, the newly reported technique called the O-FAFF can be considered (24). As described above, the omentum is harvested off the greater curvature of the stomach with preservation of the gastroepiploic vessels for microvascular anastomoses (Figure 1). The omentum is then placed within a piece of acellular dermal matrix (ADM), a commonly used biologic matrix that complements implant-based reconstruction, to provide structure to the otherwise amorphous omentum. The ADM is shaped around a breast sizer prior to placement of the omentum within the construct (Figure 2). To avoid the use of an implant or an additional muscle flap with the omentum for breast reconstruction after a nipple- or skin-sparing mastectomy, the omental volume is augmented with fat obtained by liposuction (Figure 3). Fat grafting is an established option to supplement autologous and implant-based reconstruction (25). Concurrent fat grafting provides a solution to the difficulty in predicting the size of the omentum pre-operatively. The O-FAFF construct is then suspended on the chest after microvascular anastomoses are made between the internal mammary vessels and the gastroepiploic vessels (Figure 4).
Outcome
Long-term results have reported a stable size and shape of the omentum within the reconstructed breast, even after radiation (11,17,18,23). Our experience with the O-FAFF also showed stable breast volume without fat necrosis (24). Studies evaluating patient satisfaction after undergoing laparoscopically harvested omentum for autologous breast microvascular reconstruction has shown that all patients have high satisfaction regarding breast volume (17,24).
The potential drawbacks of using the omentum include concern for iatrogenic intra-abdominal injury or future abdominal surgery. It is reassuring, however, that patients who have undergone abdominal surgery after laparoscopic omental flap surgery have had successfully completed surgeries with findings of minimal intra-abdominal adhesions (21).
Conclusions
The omentum is a viable option for autologous breast reconstruction. It is a flap that is soft, malleable, with vascular reliability, low risk of atrophy, minimal donor-site morbidity, and high patient satisfaction (17,18,23). While a pedicled omental flap has historically been used more often, an omental free flap provides more versatility for patients with fewer drawbacks. In particular, the O-FAFF may become the preferred technique because it allows the omental volume to be increased with fat grafting, while providing breast projection with the use of ADM as long as a sufficient mastectomy skin envelope is present. As more women undergo mastectomies for breast cancer or genetic predisposition, the omentum provides a natural-appearing breast reconstruction for those lacking sufficient autologous tissue from more traditional donor sites or who wish to minimize donor site morbidity.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Breast Surgery for the series “Cutting-edge of Complex Breast Reconstruction”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-109). The series “Cutting-edge of Complex Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. DHN served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Breast Surgery from November 2019 to December 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kiricuta I. The use of the great omentum in the surgery of breast cancer. Presse Med 1963;71:15-7. [PubMed]
- Di Nicola V. Omentum a powerful biological source in regenerative surgery. Regen Ther 2019;11:182-91. [Crossref] [PubMed]
- Liebermann-Meffert D. The greater omentum. Anatomy, embryology, and surgical applications. Surg Clin North Am 2000;80:275-93. [Crossref] [PubMed]
- Botianu PVH. Current indications for the intrathoracic transposition of the omentum. J Cardiothorac Surg 2019;14:103. [Crossref] [PubMed]
- Killeen S, Devaney A, Mannion M, et al. Omental pedicle flaps following proctectomy: a systematic review. Colorectal Dis 2013;15:e634-45. [Crossref] [PubMed]
- Hultman CS, Carlson GW, Losken A, et al. Utility of the omentum in the reconstruction of complex extraperitoneal wounds and defects. Ann Surg 2002;235:782-95. [Crossref] [PubMed]
- Newing RK, Pribaz JJ, Bennett RC, et al. Omental transposition and skin graft in the management of chest wall recurrence of carcinoma of the breast. Aust N Z J Surg 1979;49:546-51. [Crossref] [PubMed]
- Williams RJL, Fryatt IJC, Abbott WC, et al. Omental transposition in the treatment of locally advanced and recurrent breast cancer. Br J Surg 1989;76:559-63. [Crossref] [PubMed]
- Claro F Jr, Sarian LOZ, Pinto-Neto AM. Omentum for mammary disorders: a 30-year systematic review. Ann Surg Oncol 2015;22:2540-50. [Crossref] [PubMed]
- Jimenez AG, St. Germain P, Sirois M, et al. Free omental flap for skin-sparing breast reconstruction harvested laparoscopically. Plast Reconstr Surg 2002;110:545-51. [Crossref] [PubMed]
- Zaha H, Onomura M, Nomura H, et al. Free omental flap for partial breast reconstruction after breast-conserving surgery. Plast Reconstr Surg 2012;129:583-7. [Crossref] [PubMed]
- Zaha H. Partial breast reconstruction for the medial quadrants using the omental flap. Ann Surg Oncol 2014;21:3358. [Crossref] [PubMed]
- Shen G, Yu X. Application value of laparoscopy in radical mastectomy and omental breast reconstruction. Oncol Lett 2019;18:645-50. [Crossref] [PubMed]
- Romanini MV, Vidal C, Godoy J, et al. Laparoscopically harvested omental flap for breast reconstruction in Poland syndrome. J Plast Reconstr Aesthet Surg 2013;66:e303-9. [Crossref] [PubMed]
- Wang ZH, Xin P, Qu X, et al. Breast reconstruction using a laparoscopically harvested pedicled omental flap after endoscopic mastectomy for patients with breast cancer: an observational study of a minimally invasive method. Gland Surg 2020;9:676-88. [Crossref] [PubMed]
- Guan D, Lin H, Ly Z, et al. The oncoplastic breast surgery with pedicled omental flap harvested by laparoscopy: initial experiences from China. World J Surg Oncol 2015;13:95. [Crossref] [PubMed]
- van Alphen TC, Fechner MR, Smit JM, et al. The laparoscopically harvested omentum as a free flap for autologous breast reconstruction. Microsurgery 2017;37:539-45. [Crossref] [PubMed]
- Zaha H, Inamine S, Naito T, et al. Laparoscopically harvested omental flap for immediate breast reconstruction. Am J Surg 2006;192:556-8. [Crossref] [PubMed]
- Sinelnikov MY, Chen K, Sukorceva NS, et al. A Clinical Case of Breast Reconstruction with Greater Omentum Flap for Treatment of Upper Extremity Lymphedema. Plast Reconstr Surg Glob Open 2019;7:e2402 [Crossref] [PubMed]
- Li N, Zheng Z, Li J, et al. Immediate breast reconstruction with omental flap for luminal breast cancer patients. Medicine 2017;96:33:e7797.
- Zaha H, Abe N, Sagawa N, et al. Oncoplastic surgery with omental flap reconstruction: a study of 200 cases. Breast Cancer Res Treat 2017;162:267-74. [Crossref] [PubMed]
- Shash H, Al-halabi B, Aldekhayel S, et al. Laparoscopic harvesting of omental flaps for breast reconstruction- a review of the literature and outcome analysis. Plast Surg (Oakv) 2018;26:126-33. [Crossref] [PubMed]
- Ni C, Zhu Z, Xin Y, et al. Oncoplastic breast reconstruction with omental flap: A retrospective study and systematic review. J Cancer 2018;9:1782-90. [Crossref] [PubMed]
- Nguyen DH, Ma IT, Choi YK, et al. Creating a Biological Breast Implant with an Omental Fat-Augmented Free Flap (O-FAFF). Barcelona Breast Annual Meeting 2020, Barcelona, Spain.
- Katzel EB, Bucky LP. Fat grafting to the breast: clinical applications and outcomes for reconstructive surgery. Plast Reconstr Surg 2017;140:69S-76S. [Crossref] [PubMed]
Cite this article as: Ma IT, Deptula P, Nguyen DH. Evolution in breast reconstruction using the omentum. Ann Breast Surg 2021;5:18.

