
Advocating for closer collaboration in breast surgery and breast reconstruction: a narrative review
Introduction
The modern age has seen a global rise in oncoplastic breast-conserving surgery for breast cancer patients. For those patients who are not candidates for breast conservation, post-mastectomy breast reconstruction has become increasingly prominent compared to mastectomy alone (1,2). A study conducted in 2017 in China by the China Anti-Cancer Association and the Chinese College of Surgeons revealed that of the 110 surveyed institutional hospitals, 87.3% had performed breast reconstructions (3). In 2017, 10.7% of the post-mastectomy patients received breast reconstruction, 67.6% of which were immediate, and 65% of which were implant-based reconstructions.
According to the same survey, approximately 40% of the total breast conserving cases were oncoplastic, and 15% of the oncoplastic cases were partial breast reconstructions (4). Given that breast conservation and oncoplastic reconstruction are being increasingly favored in China, there is a higher demand for physicians to have expertise in breast cancer treatment, breast oncoplasty, and breast reconstruction. Consequently, a closer multidisciplinary collaboration among different specialties is critical to providing optimal care for patients. From the oncological perspectives, we summarized the primary issues that could promote better collaboration and improve surgical outcomes. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/abs-20-64).
Methods
A MEDLINE (PubMed) search for articles published between January 2001 and April 2020 was performed using a combination of the following keywords: “breast reconstruction”, “breast surgery”, “breast cancer”, and “China” or “Chinese”. Articles were limited to English or Chinese language. Titles, abstracts, and full texts, if available, were scrutinized by the two authors of this review. Studies pertaining to the clinical research on breast reconstruction in the Chinese population in mainland China were included. Review articles, clinical research studies on non-mainland Chinese databases/populations, and benchwork studies were excluded. Relevant studies were classified as trend studies, surgical technique-related, or diagnostic/adjuvant therapy-related. Studies with more than 6 months of follow-up were noted as reporting long-term outcomes.
Results
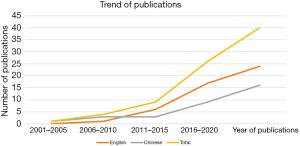
There were a total of 60 potential citations from the MEDLINE search, and 40 clinical studies with data collected on patients from mainland China were reviewed. The number of publications in both the English and Chinese languages has nearly tripled over the past 5 years (Figure 1). Of the 40 studies, 11 were trend/survey analyses on breast reconstruction, 9 were related to reconstructive surgical techniques, 5 were related to diagnostic measures or adjuvant treatment, and 25 reported long-term aesthetic, oncological, or quality of life outcomes.
Discussion
Incision design
The art of surgical planning and execution comprise both oncological and plastic considerations, and poses challenges to surgeons. For the benefits of postoperative aesthetics, nipple-sparing mastectomy (NSM)/skin-sparing mastectomy (SSM) have been shown to be superior and are the preferred options for many breast surgeons. Given the demonstrated oncologic safety of both NSM and SSM, breast surgeons frequently design incisions in locations that are well concealed but that also allow sufficient access and exposure to perform the mastectomy.
An adequate incision design facilitates proper mastectomy and preserves maximal flap perfusion. An additional axillary incision can be performed if an axillary dissection is needed to minimize traumatic traction on the mastectomy skin flaps. A wide variety of incisions have been described, including peri-areolar, circum-areolar, lateral, inframammary fold, radial, mastopexy/reduction, and endoscopic incisions, and there is no consensus as to the best choice. A recent systematic review on NSM incisions for patients undergoing immediate reconstruction revealed a shift towards the inframammary (37.8%) incision followed by radial (37.2%), and peri-areolar incisions (15.2%) (5). Although offering easy access for the mastectomy along with well-hidden scars, a pooled rate analysis showed peri-areolar incisions had the highest risk for nipple-areolar complex (NAC) necrosis, reaching an incidence of nearly 20% (5), posing the subsequent reconstruction at higher risk. To combat this, peri-areolar combined with radial incisions have often been used in our practice for patients with an NAC diameter smaller than 3.5 cm, with intraoperative indocyanine green (ICG) imaging of the NAC and the mastectomy flap being routinely used to assess adequate perfusion. Reduction incisions have been found to be the preferred choices for oncoplastic breast-conserving surgery and in circumstances when breast conservation is performed in a pendulous, ptotic breast. Skin-reducing techniques that preserve the NAC and sufficient breast skin are our preferred choices for oncoplastic reconstruction (6-8).
Mastectomy flap perfusion
Maintaining adequate mastectomy flap perfusion can reduce the risk of flap necrosis that leads to reconstructive failure and the potential delay of adjuvant therapies. Previous studies have used preoperative magnetic resonance imaging (MRI) to evaluate mastectomy skin flap thickness and observed that a NSM flap thickness smaller than 8 mm increased the risk of ischemic events (9). However, a thicker mastectomy flap may contain residual breast tissue that can increase the risk of local recurrence. A flap thicker than 5 mm was found to significantly increase the prevalence of residual breast tissue (10). Other studies have found skin flaps used during a NSM and prophylactic mastectomy are associated with the presence of residual breast tissue (11). Again, a delicate balance exists between performing a sound oncologic mastectomy and minimizing ischemic injury to the skin flaps that can compromise the final aesthetic result.
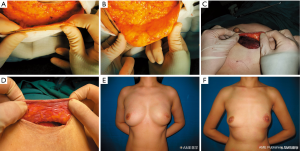
Anatomical studies of the breast have revealed a superficial fascial system encasing the corpus mammae, with the Cooper’s ligament going through the fascial system, connecting the deep fascia and the dermis (12). Beer et al. reported that no breast tissue is present superficial to the superficial fascial layer; however, not all breasts possess a superficial fascial layer, and the distance between the dermis and this layer can vary between 0.2 and 4 mm (13). Larson et al., on the other hand, found that the subcutaneous tissue between the dermis and the breast parenchyma had a median thickness of 10 mm; however, no correlations were found between the thickness of the subcutaneous tissue and body mass index, age, breast specimen weight, or the subcutaneous thickness of the contralateral breast (14). The discrepancies between these studies could be attributed to the different quadrants of the mastopexy sampling. From the authors’ experience in Chinese patients, there is tremendous variability of subcutaneous thickness between individuals or from one breast to the other. In our practice of immediate reconstruction for breast cancer patients, we routinely keep a thin mastectomy flap (Figure 2), and we feel a 1 cm median mastectomy flap thickness should be managed with caution. It is possible that ethnic differences exist across Asian and Western populations in terms of subcutaneous tissue thickness; however, more studies are necessary to determine an ideal flap thickness to suit the Chinese population.
Preservation of collateral vessels
Post-mastectomy lymphedema can adversely affect patients’ arm function and quality of life. Studies have shown that patients who undergo axillary lymph node dissection have four times the risk of developing lymphedema than those who have sentinel lymph node biopsy (15). Recently, super-microsurgical repair of the lymphatic system has become increasingly prevalent and is now considered the standard treatment at many institutions, with excellent outcomes reported in long-term follow-ups (16,17). Several studies have reported prophylactic surgery performed concurrently with an axillary lymph node dissection can also reduce the risk of secondary lymphedema (18-20) with a median reduced life-time cost of approximately 45% for the healthcare system (21). ICG fluorescent imaging or blue dye (18,22) is used to trace lymphatic vessels using reverse axillary mapping, and end-to-end, end-to-side, or octopus anastomoses can be performed to restore drainage through the axilla. The orientation of the anastomoses are based on the number, size, and pressure of the lymphatic and recipient veins (22-25). Most authors report use of collateral branches of the axillary vessels such as the lateral thoracic vessels or branches of the thoracodorsal vessels as recipients for lymphovenous bypass or for vascularized free lymph node transfer. Preservation of these vessels at the time of axillary lymph node dissection by breast surgeons could facilitate the subsequent anastomosis.
Timing of reconstruction and radiation
Radiation is an essential therapy in the comprehensive treatment for breast cancer and can significantly reduce local recurrence and improve overall survival (26). However, radiation can lead to inflammation and fibrotic changes to the tissue and have adverse effects on the reconstructed breast (26,27). The timing and the type of breast reconstruction are important considerations for resection and reconstructive surgery when post-operative radiation is indicated.
Autologous reconstruction is generally recommended and is considered the gold standard for breast reconstruction in patients receiving chest wall radiation, as implant-based reconstruction is fraught with higher morbidity and complications rates (28-31). Multiple studies have reported that the sequence of radiation and autologous reconstruction has no impact on the overall occurrence of complications (32-35), while other researchers have found that radiation poses higher risks for fat necrosis in autologous reconstruction (36-38). This is a serious issue that can cause anxiety for the patient and physician alike, eliciting the potential differential diagnosis for local recurrence during follow-ups. In addition, radiation can induce flap shrinkage leading to noticeable asymmetry, and in severe cases it may require further surgical interventions (38,39).
Radiation has been shown to increase grade III–IV capsular contracture to 32% and reconstruction failure to 20% in prosthesis device-based reconstruction (40-42). Two-staged prosthesis-based reconstruction is widely employed in most medical centers internationally. The exchange from tissue expander (TE) to permanent implant (PI) can be performed before or after radiation. There is a general consensus that radiation on TE increases the risk for prosthesis loss compared with radiation on PI (22.9% vs. 5.6%) (43). On the other hand, radiation on the PI can increase the risk for capsular contracture, compared with radiation on TE. However, the lower risk of capsular contracture using a two-stage approach may be due to the opportunity to perform capsulotomies and capsulectomy at the time of exchange after radiation. Direct-to-implant (DTI) reconstructions with a pedicled latissimus dorsi flap or bio-prosthetic meshes have drawn increasing attention, but have produced similar complications and outcomes compared with two-staged TE/PI reconstruction (44-46). In our cancer center, the majority of the implant-based reconstructions are DTI, as it can obviate the need for expansion and a secondary operation for exchanging the expander for a PI. Other factors such as insurance coverage and access to prosthetic materials are also factors that we consider in our algorithmic approach to reconstruction. In general, the authors also favor the use of textured anatomic silicone implants compared to smooth round implants. Several studies have compared the impact of post-mastectomy radiation on DTI reconstruction and two-staged TE/PI reconstruction and concluded that radiation poses a higher risk for complications in TE/PI reconstruction than in DTI reconstruction (47-49). We advocate durable, viable coverage of the implant to reduce the risk of reconstructive failure in patients requiring postoperative radiation. Again, a balance in performing a complete mastectomy that leaves no residual breast tissue while maintaining viable perfusion of the mastectomy skin flap is critical to achieving this objective. More studies are necessary to delineate the suitable population for DTI, especially in setting the level of radiation.
Plane of prosthesis insertion
With the advances of NSM/SSM and the advent of prosthesis and biomaterials, prepectoral breast reconstruction is gaining popularity, as it is able to maintain the reconstructed breast in the original anatomical space. In either the total or partial subpectoral technique, insertion of the prosthesis requires the elevation of muscle, which can lead to animation deformity, muscle spasm, and pain (50-54). Early prepectoral implant placement directly underneath the mastectomy flap is fraught with higher complications due to inadequate soft-tissue coverage. The application of acellular dermal matrix can provide a layer of soft tissue support and yield a higher success rate.
Numerous retrospective articles have compared the complications, oncological safety, quality of life, and pain score between prepectoral and subpectoral prosthetic breast reconstruction. While not increasing the risk for local recurrence (55), the most common complications in prepectoral prosthesis reconstruction are rippling, followed by seroma and skin flap necrosis (56). Some meta-analyses have shown that prepectoral prosthesis placement significantly reduces the odds of capsular contracture by half when compared with subpectoral placement (56,57). In the setting of pre- or postoperative radiation, the odds are further reduced to one-fourth (58). Other meta-analyses have revealed similar results related to implant loss, but results concerning the risk of skin flap necrosis are more controversial (54,56,58). The authors suggest careful patient selection and utilizing ICG imaging to ensure adequate mastectomy flap perfusion, especially in the pre-pectoral setting, to minimize the occurrence of post-operative complications. Further prospective studies of large cohorts on the Chinese population will provide more evidence to aid surgeons in proper patient selection and decision-making.
Establishment of oncoplastic teams
Surgical planning and treatment for breast cancer patients are best performed with multidisciplinary collaboration involving oncologists, plastic surgeons, radiologists, and radiation oncologists. The current treatment modality demands more refined and holistic surgical skills. In countries such as the United States and Canada, the oncological procedure is performed by surgical oncologists, and the subsequent reconstruction is performed by plastic surgeons (59,60). This approach ensures that the surgery is performed by experts in the respective fields; however, it requires coordination of physicians and access to medical services. Consequently, a surgery team with low volume and limited experience may see a higher frequency of complications, such as mastectomy skin necrosis and infection (61). A different approach combines oncological and plastic training, allowing one surgeon to complete the resection and reconstruction. Although it is more time-consuming and demands more resources, this single oncoplastic modality is gaining more popularity across the globe (62,63).
A survey conducted in 2017 in China reported that 77.8% of the hospitals containing both breast surgery and plastic surgery departments made collaborative efforts on breast surgery and reconstruction (3). Despite the high prevalence of this model, since 2012, an increasing number of hospitals have established breast oncoplastic departments, recruiting physicians who have both oncologic and plastic reconstructive surgical training. This new modality has proven to be more efficient and has helped increase the awareness of breast reconstruction in Chinese patients and the rate of immediate reconstruction in this population.
Conclusions
The treatment of breast surgery and restoration of breast aesthetics for breast cancer patients is a combination of art and science demanding careful preoperative planning, meticulous technique, and refined surgical skills. A holistic understanding of the breast cancer treatment and oncoplastic procedures ensures successful and reproducible treatment outcomes and reduces complications. While resection and reconstruction can be performed by separate independent services, a dual-trained oncoplastic reconstructive surgeon can also achieve excellent results. Regardless of which model is adopted, the establishment of oncoplastic teams is urgently needed in Chinese medical centers to provide high quality multidisciplinary care to breast cancer patients.
While this narrative overview has hopefully provided an illuminating perspective, it may be limited by the unsystematic nature of its design, and more qualitative and quantitative systematic reviews of high-level clinical studies specific to the Chinese population are warranted.
Acknowledgments
Funding: Tianjin “the Belt and Road” Technological Innovation and Cooperation Grant (no. 18PTZWHZ00050) and the Sino-Russian Joint Research Center for Oncoplastic Breast Surgery.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Edward I. Chang) for the series “Novel Innovations and Advancements in Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-64
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-64). The series “Novel Innovations and Advancements in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Driul L, Bernardi S, Bertozzi S, et al. New surgical trends in breast cancer treatment: conservative interventions and oncoplastic breast surgery. Minerva Ginecol 2013;65:289-96. [PubMed]
- Jonczyk MM, Jean J, Graham R, et al. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res Treat 2019;173:267-74. [Crossref] [PubMed]
- Xiu BQ, Guo R, Yang BL, et al. Current trends of breast reconstruction after mastectomy in China: a cross-sectional study. Zhonghua Zhong Liu Za Zhi 2019;41:546-51. [PubMed]
- Shao D, Su Y, Xiu B, et al. Oncoplastic breast conserving surgery: a cross-sectional study of 110 breast surgery centers in China. Chinese Journal of Practical Surgery 2019;39:1176-80.
- Daar DA, Abdou SA, Rosario L, et al. Is There a preferred incision location for nipple-sparing mastectomy? a systematic review and meta-analysis. Plast Reconstr Surg 2019;143:906e-19e. [Crossref] [PubMed]
- Dietz J, Fedele G. Skin reduction nipple-sparing mastectomy. Ann Surg Oncol 2015;22:3404. [Crossref] [PubMed]
- Kontos M, Lanitis S, Constantinidou A, et al. Nipple-sparing skin-reducing mastectomy with reconstruction for large ptotic breasts. J Plast Reconstr Aesthet Surg 2020;73:690-5. [Crossref] [PubMed]
- Pontell ME, Saad N, Brown A, et al. Single stage nipple-sparing mastectomy and reduction mastopexy in the ptotic breast. Plast Surg Int 2018;2018:9205805 [Crossref] [PubMed]
- Frey JD, Salibian AA, Choi M, et al. Mastectomy flap thickness and complications in nipple-sparing mastectomy: objective evaluation using magnetic resonance imaging. Plast Reconstr Surg Glob Open 2017;5:e1439 [Crossref] [PubMed]
- Tokin C, Weiss A, Wang-Rodriguez J, et al. Oncologic safety of skin-sparing and nipple-sparing mastectomy: a discussion and review of the literature. Int J Surg Oncol 2012;2012:921821 [Crossref] [PubMed]
- Giannotti DG, Hanna SA, Cerri GG, et al. Analysis of skin flap thickness and residual breast tissue after mastectomy. Int J Radiat Oncol Biol Phys 2018;102:82-91. [Crossref] [PubMed]
- Rehnke RD, Groening RM, Van Buskirk ER, et al. Anatomy of the superficial fascia system of the breast: a comprehensive theory of breast fascial anatomy. Plast Reconstr Surg 2018;142:1135-44. [Crossref] [PubMed]
- Beer GM, Varga Z, Budi S, et al. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer 2002;94:1619-25. [Crossref] [PubMed]
- Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg 2011;127:27-33. [Crossref] [PubMed]
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. [Crossref] [PubMed]
- Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2013;132:1305-14. [Crossref] [PubMed]
- Engel H, Lin CY, Huang JJ, et al. Outcomes of lymphedema microsurgery for breast cancer-related lymphedema with or without microvascular breast reconstruction. Ann Surg 2018;268:1076-83. [Crossref] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery 2014;34:421-4. [Crossref] [PubMed]
- Feldman S, Bansil H, Ascherman J, et al. Single institution experience with lymphatic microsurgical preventive healing approach (LYMPHA) for the primary prevention of lymphedema. Ann Surg Oncol 2015;22:3296-301. [Crossref] [PubMed]
- Jørgensen MG, Toyserkani NM, Sørensen JA. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: a systematic review and meta-analysis. Microsurgery 2018;38:576-85. [Crossref] [PubMed]
- Squitieri L, Rasmussen PW, Patel KM. An economic analysis of prophylactic lymphovenous anastomosis among breast cancer patients receiving mastectomy with axillary lymph node dissection. J Surg Oncol 2020;121:1175-8. [Crossref] [PubMed]
- Chang EI, Skoracki RJ, Chang DW. Lymphovenous anastomosis bypass surgery. Semin Plast Surg 2018;32:22-7. [Crossref] [PubMed]
- Chen WF, Yamamoto T, Fisher M, et al. The "octopus" lymphaticovenular anastomosis: evolving beyond the standard supermicrosurgical technique. J Reconstr Microsurg 2015;31:450-7. [Crossref] [PubMed]
- Narushima M, Mihara M, Yamamoto Y, et al. The intravascular stenting method for treatment of extremity lymphedema with multiconfiguration lymphaticovenous anastomoses. Plast Reconstr Surg 2010;125:935-43. [Crossref] [PubMed]
- Yamamoto T, Yoshimatsu H, Yamamoto N, et al. Side-to-end lymphaticovenular anastomosis through temporary lymphatic expansion. PLoS One 2013;8:e59523 [Crossref] [PubMed]
- Rozen WM, Ashton MW. Radiotherapy and breast reconstruction: oncology, cosmesis and complications. Gland Surg 2012;1:119-27. [PubMed]
- Straub JM, New J, Hamilton CD, et al. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol 2015;141:1985-94. [Crossref] [PubMed]
- Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat 2011;127:15-22. [Crossref] [PubMed]
- Jagsi R, Momoh AO, Qi J, et al. Impact of radiotherapy on complications and patient-reported outcomes after breast reconstruction. J Natl Cancer Inst 2018;110:157-65. [Crossref] [PubMed]
- Kronowitz SJ. Current status of implant-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg 2012;130:513e-23e. [Crossref] [PubMed]
- Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg 2012;130:282-92. [Crossref] [PubMed]
- Berbers J, van Baardwijk A, Houben R, et al. 'Reconstruction: before or after postmastectomy radiotherapy?' A systematic review of the literature. Eur J Cancer 2014;50:2752-62. [Crossref] [PubMed]
- Schaverien MV, Macmillan RD, McCulley SJ. Is immediate autologous breast reconstruction with postoperative radiotherapy good practice?: a systematic review of the literature. J Plast Reconstr Aesthet Surg 2013;66:1637-51. [Crossref] [PubMed]
- Billig J, Jagsi R, Qi J, et al. Should immediate autologous breast reconstruction be considered in women who require postmastectomy radiation therapy? A prospective analysis of outcomes. Plast Reconstr Surg 2017;139:1279-88. [Crossref] [PubMed]
- Kelley BP, Ahmed R, Kidwell KM, et al. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: are current practices ideal? Ann Surg Oncol 2014;21:1732-8. [Crossref] [PubMed]
- He S, Yin J, Robb GL, et al. Considering the optimal timing of breast reconstruction with abdominal flaps with adjuvant irradiation in 370 consecutive pedicled transverse rectus abdominis myocutaneous flap and free deep inferior epigastric perforator flap performed in a Chinese oncology center: is there a significant difference between immediate and delayed? Ann Plast Surg 2017;78:633-40. [Crossref] [PubMed]
- Khansa I, Momoh AO, Patel PP, et al. Fat necrosis in autologous abdomen-based breast reconstruction: a systematic review. Plast Reconstr Surg 2013;131:443-52. [Crossref] [PubMed]
- Mirzabeigi MN, Smartt JM, Nelson JA, et al. An assessment of the risks and benefits of immediate autologous breast reconstruction in patients undergoing postmastectomy radiation therapy. Ann Plast Surg 2013;71:149-55. [Crossref] [PubMed]
- Tran NV, Chang DW, Gupta A, et al. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg 2001;108:78-82. [Crossref] [PubMed]
- Lam TC, Borotkanics R, Hsieh F, et al. Immediate two-stage prosthetic breast reconstruction failure: radiation is not the only culprit. Plast Reconstr Surg 2018;141:1315-24. [Crossref] [PubMed]
- Fowble B, Park C, Wang F, et al. Rates of reconstruction failure in patients undergoing immediate reconstruction with tissue expanders and/or implants and postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 2015;92:634-41. [Crossref] [PubMed]
- Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol 2014;21:118-24. [Crossref] [PubMed]
- Lam TC, Hsieh F, Boyages J. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: a systematic review. Plast Reconstr Surg 2013;132:511-8. [Crossref] [PubMed]
- Agafonoff S, Kundu N, Schwarz G, et al. Immediate implant reconstruction in patients undergoing radiation therapy: opportunities and challenges. Ann Surg Oncol 2020;27:963-5. [Crossref] [PubMed]
- Doherty C, Pearce S, Baxter N, et al. Trends in immediate breast reconstruction and radiation after mastectomy: a population study. Breast J 2020;26:446-53. [Crossref] [PubMed]
- Srinivasa DR, Garvey PB, Qi J, et al. Direct-to-implant versus two-stage tissue expander/implant reconstruction: 2-year risks and patient-reported outcomes from a prospective, multicenter study. Plast Reconstr Surg 2017;140:869-77. [Crossref] [PubMed]
- Dicuonzo S, Leonardi MC, Radice D, et al. Long-term results and reconstruction failure in patients receiving postmastectomy radiation therapy with a temporary expander or permanent implant in place. Plast Reconstr Surg 2020;145:317-27. [Crossref] [PubMed]
- Naoum GE, Salama L, Niemierko A, et al. Single stage direct-to-implant breast reconstruction has lower complication rates than tissue expander and implant and comparable rates to autologous reconstruction in patients receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys 2020;106:514-24. [Crossref] [PubMed]
- Lin AM. The Impact of post-mastectomy radiation therapy on permanent implants in direct-to-implant breast reconstruction versus tissue expanders in two-stage breast reconstruction. Plast Reconstr Surg Glob Open 2018;6:2. [Crossref]
- Ribuffo D, Berna G, De Vita R, et al. Dual-plane retro-pectoral versus pre-pectoral dti breast reconstruction: an Italian multicenter experience. Aesthetic Plast Surg 2021;45:51-60. [Crossref] [PubMed]
- Kokosis G, Dayan JH. Correction of nipple-areola complex malposition with conversion from subpectoral to prepectoral plane: proof of concept. Plast Reconstr Surg 2020;146:237e-8e. [Crossref] [PubMed]
- Holland MC, Lentz R, Sbitany H. Surgical correction of breast animation deformity with implant pocket conversion to a prepectoral plane. Plast Reconstr Surg 2020;145:632-42. [Crossref] [PubMed]
- Jones GE, King VA, Yoo A. Prepectoral site conversion for animation deformity. Plast Reconstr Surg Glob Open 2019;7:e2301 [Crossref] [PubMed]
- Li L, Su Y, Xiu B, et al. Comparison of prepectoral and subpectoral breast reconstruction after mastectomies: a systematic review and meta analysis. Eur J Surg Oncol 2019;45:1542-50. [Crossref] [PubMed]
- Nealon KP, Weitzman RE, Sobti N, et al. Prepectoral direct-to-implant breast reconstruction: safety outcome endpoints and delineation of risk factors. Plast Reconstr Surg 2020;145:898e-908e. [Crossref] [PubMed]
- Abbate O, Rosado N, Sobti N, et al. Meta-analysis of prepectoral implant-based breast reconstruction: guide to patient selection and current outcomes. Breast Cancer Res Treat 2020;182:543-54. [Crossref] [PubMed]
- Li Y, Xu G, Yu N, et al. Prepectoral versus subpectoral implant-based breast reconstruction: a meta-analysis. Ann Plast Surg 2020;85:437-47. [Crossref] [PubMed]
- Sobti N, Weitzman RE, Nealon KP, et al. Evaluation of capsular contracture following immediate prepectoral versus subpectoral direct-to-implant breast reconstruction. Sci Rep 2020;10:1137. [Crossref] [PubMed]
- Peiris L, Olson D, Kelly D. Oncoplastic and reconstructive breast surgery in Canada: breaking new ground in general surgical training. Can J Surg 2018;61:294-9. [Crossref] [PubMed]
- Losken A, Kapadia S, Egro FM, et al. Current opinion on the oncoplastic approach in the USA. Breast J 2016;22:437-41. [Crossref] [PubMed]
- Gfrerer L, Mattos D, Mastroianni M, et al. Assessment of patient factors, surgeons, and surgeon teams in immediate implant-based breast reconstruction outcomes. Plast Reconstr Surg 2015;135:245e-52e. [Crossref] [PubMed]
- Down SK, Pereira JH, Leinster S, et al. Training the oncoplastic breast surgeon-current and future perspectives. Gland Surg 2013;2:126-7. [PubMed]
- Sandelin K, King E, Redman S. Breast reconstruction following mastectomy: current status in Australia. ANZ J Surg 2003;73:701-6. [Crossref] [PubMed]
(English Language Editor: J. Gray)
Cite this article as: He S, Yin J. Advocating for closer collaboration in breast surgery and breast reconstruction: a narrative review. Ann Breast Surg 2021;5:31.

