
Techniques for overcoming a missing clip during pre-operative needle localization for lumpectomy: case report
Introduction
A landmark study in 1985 validated the utility and safety of breast conservation therapy (BCT) for early stage breast cancers (1). The investigators proved an equivalence in oncologic outcomes when comparing BCT versus total mastectomy. Several years later, BCT gained notable traction in clinical practice with the endorsement of the National Institutes of Health (2). Now with corroborating long-term follow-up data, BCT has become the standard of care in the treatment of early stage breast cancers (3).
BCT consists of partial mastectomy, commonly referred to as lumpectomy, followed by adjuvant radiation. The conservative approach in surgical dissection inherent to lumpectomy leads to improved cosmesis and less wound morbidity (4). A typical course in patient management begins with a tissue diagnosis of an early stage breast lesion. In recent years, stereotactic-guided vacuum-assisted core-needle biopsy (SVCBx) has become a widely accepted technique for sampling non-palpable breast lesions (5). Compared to surgical biopsy, SVCBx is associated with a higher rate of negative margins as well as lower disease-related morbidity and overall costs to the healthcare system (6). Once a tissue diagnosis has been obtained, patients are typically referred to a surgeon who will ultimately be responsible for choosing appropriate candidates for BCT.
Successfully removing non-palpable breast lesions while avoiding damage to the surrounding healthy tissue requires direction. In order to guide surgeons in their dissection, a percutaneous wire is commonly placed in the preoperative setting. This technique is known as wire-guided localization (WGL) and has become a widely accepted technique for surgeons to navigate lumpectomy dissections (7). A wire can be placed under the guidance of prior imaging as well as the characteristics of the target lesions themselves at the time of localization (8). Furthermore, wire placement is routinely aided by fiduciary clips left near the target lesion at the time of a previous biopsy. While this process proves highly efficient and accurate when all elements function expectantly, WGL does not come without its own unique challenges. Clip migration and intra-operative loss are some of the more well-known potential challenges physicians may encounter when treating patients with BCT (9). Clip migration has the potential to mislead treatment both in terms of surgery and radiation. There is also the possibility that lifelong surveillance will be affected if the clip migrates far enough from the lesion’s true location. Fiduciary markers may also be misplaced during surgery. The inability to confirm that the marker has been removed within the lumpectomy sample can cause the surgeon much distress and, if one can imagine, results in a blind search for the “needle in the haystack”.
Despite several well-known potential dilemmas involving fiduciary markers, to our knowledge, there has been no discussion in the literature in regard to an inability to locate markers in the immediate pre-operative setting. In the present article, we describe a case in which preoperative WGL could not performed due to an inability to visualize the fiduciary clip. Furthermore, we share details of how a multidisciplinary team was able to collaborate under pressure to quickly overcome this obstacle and successfully perform a “blind lumpectomy”.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/abs-20-112).
Case presentation
A 60-year-old female was referred to the surgery clinic for evaluation of a new left breast lesion. She had no symptoms and her medical history was unremarkable. She had undergone routine screening mammography since the age of 45 with no significant findings. She had no family history of cancer. On physical exam her breast were symmetrical with no nipple discharge or skin changes. There were no palpable lesions in either breast or axillae.
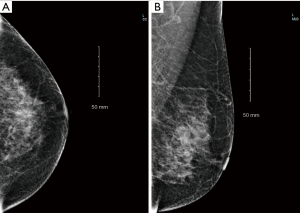
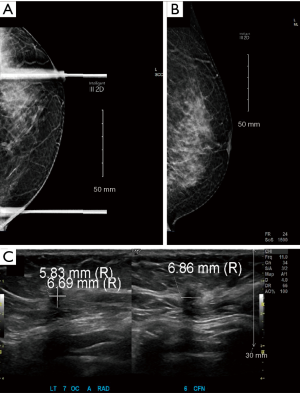
Several weeks prior, the patient had undergone screening mammography that demonstrated a new pleomorphic nodule deep within her left breast measuring less than 1 cm, reported as Breast Imaging-Reporting and Data System-0 (BIRADS-0, Figure 1). Subsequent diagnostic mammography and ultrasound were notable for an irregular nodule measuring 6×7 mm located at the 7 o’clock position of the left breast 6 cm from the nipple, reported as BIRADS-4 (Figure 2). She then underwent SVCBx with fiduciary clip placement. Post-biopsy imaging revealed that the lesion had been completely removed. Although the clip was only partially visible in the mediolateral view (ML) due to the depth of the lesion, it was clearly identifiable in the craniocaudal projection (CC, Figure 3). Final pathology of the biopsy reported atypical ductal hyperplasia with the caveat that cytokeratin 5/6 and estrogen receptor expression could not be evaluated due to fragmentation and small sample size. Excisional biopsy was therefore recommended to exclude more insidious lesions such as ductal carcinoma in situ. After discussing the available options with the patient, she elected to undergo lumpectomy with preoperative stereotactic WGL.

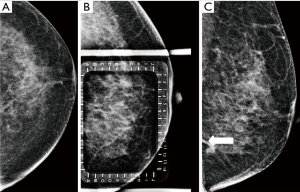
The patient was sent to the radiology department on the morning of surgery. Stereotactic WGL was then attempted with the aid of a localization grid. However, despite several attempts using various techniques and projections, the clip could not be visualized. Localization under ultrasound guidance was then attempted but the clip was indistinguishable from the surrounding fibroglandular tissue. Furthermore, there were no identifiable remnants of the prior lesion to help guide in the localization. The surgical team was notified of the inability to visualize the clip. After multidisciplinary discussion, it was decided to attempt localization using a standard mammogram technique without a localization grid. After multiple failed attempts using different projections, the clip was partially visualized in the ML view (Figure 4). While this was somewhat reassuring, the clip’s partial view did not allow for completion of WGL. After deliberation under the pressure of time lost, we devised a method to guide the dissection without the aid of a wire. Taking into account the intersection of breast tissue with the chest wall in the image with partial clip visualization, an estimate of the angular position of the clip was obtained. We then estimated of the clip’s distance from the nipple utilizing measurements from the patient’s previous imaging. By combining these measurements we then triangulated an estimate of the clip’s position and marked the patient’s skin accordingly (Figure 5).
Utilizing the skin markings for guidance, the patient underwent a standard lumpectomy. The first tissue sample was sent to radiology, but the clip was not visualized on X-ray. The patient’s prior imaging was reviewed intra-operatively, and after further judicious dissection, a second sample was sent. Unfortunately, the clip was not found in this sample either. Finding ourselves at a lost, we then consulted the radiologist intra-operatively in hopes that they may have another idea. It was then proposed that we try using fluoroscopy to locate the clip. Moments later, with the aid of a C-arm, the clip was finally identified. A hemostat was then used to mark the clip’s position in the surgical field. The dissection proceeded using the hemostat for guidance and a third sample was sent to radiology. With this sample the clip was successfully identified (Figure 6). The surgery then proceeded with closing the wound with particular focus towards achieving favorable cosmesis. Although several samples had been taken in attempts to retrieve the clip, the samples were small enough that the final wound cavity was left with ample surrounding breast tissue to offer for a comfortable oncoplastic reconstruction. Several planes of healthy breast tissue were created to overlap the wound bed in a fashion that complimented the breast’s natural contour. The flaps were then secured into place using absorbable suture. With a cosmetically appealing oncoplastic reconstruction, the surgery concluded and the patient was discharged home later that day.
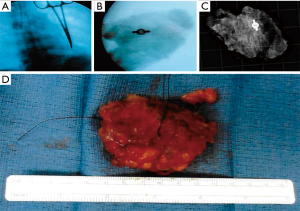
The lumpectomy specimens were negative for carcinoma. The reported findings were convoluted, however, consisting of atypical ductal hyperplasia, intraductal papilloma, fibroadenoma, radial scaring, and complexed sclerosing adenosis. The patient was counseled and chose not to pursue chemoprevention. At the time of this writing, over the course of a 6-month follow-up, the patient has remained in good spirits and highly satisfied with both her oncologic and cosmetic outcomes.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
This case demonstrates some of the potential pitfalls that can occur when relying on fiduciary clips. Although several other problems involving fiduciary clips have been described, to our knowledge this is the first article to address the challenge that arises when a clip cannot be identified in the immediate pre-operative setting for elective lumpectomy. As described in this patient’s case, the inability to identify the marking clip results in a major dilemma since WGL cannot be completed just prior to surgery. With non-palpable breast lesions, it is nearly impossible to perform a breast-conserving resection without intra-operative guidance. As such, the unexpected failure of pre-operative WGL can be a distressing situation for surgeons. Aborting the procedure and re-scheduling is an acceptable option, however, this may cause undue stress to the patient and further exacerbate any anxiety they may already have regarding breast surgery. On the other hand, one may choose to persevere without WGL at the risk of performing a mediocre lumpectomy. In the case presented, we chose to proceed with a “blind lumpectomy”, and were ultimately successful in our endeavor with the help of intra-operative fluoroscopy and multidisciplinary collaboration.
BCT has become the standard of care in the treatment of low-stage breast lesions with fiduciary clips serving a key role. Given that SVCBx removes all radiographic evidence of lesions in over 50% of cases, these markers serve a critical role as they are often the only reference point to the lesion in question (10). While the benefits are undoubted, clip migration remains a significant challenge when relying on fiduciary markers. Clip migration, also known as clip displacement, simply describes when the position of a clip marker has changed. Several mechanisms for clip migration have been proposed, some of which include hematoma formation, scarring, variations in breast composition, lack of real-time visualization during clip deployment, and the “accordion effect” (11). The accordion effect is a mechanism for displacement commonly associated with clips placed under stereotactic guidance. While breast tissue is compressed, a biopsy is obtained, and a clip subsequently placed under stereotactic guidance. When the breast tissue is released from compression, force may then be exerted on the clip, thus resulting in displacement. It is estimated that displacement occurs in up to 25% of all clip deployments with incidence positively correlating with time between deployment and subsequent imaging (12). Moreover, the migratory distance from the target lesion is more than 1.0 cm in the majority of cases. This discrepancy can be significant given that lumpectomy comes with the intention of removing the least amount of tissue necessary to complete an oncologic resection. Physicians caring for patients with breast disease should be aware that there is a 1 in 4 chance that their patient’s clip could be 1.0 cm or more off target.
It is imperative that post-procedural mammograms be obtained after biopsy and marker placement. These images serve to record the clips position in relation to the target lesion as well as any other identifiable landmarks. Additional imaging is necessary at the time of any future procedures to confirm that the clip remains in its original location or to document that it has migrated. At the time of surgery, X-rays are typically obtained intra-operatively in order to confirm that the marker has been successfully removed with the specimen. Great care must be taken during a lumpectomy dissection, not only to remove the least amount of tissue necessary to achieve adequate margins, but also to avoid inadvertent displacement of the wire or removal of the marking clip. In particular, suction must be used judiciously, as it is possible to remove the clip via the suctioning system. If such an event occurs unknowingly, surgical morbidity is likely to increase as unnecessary tissue is removed in attempts to retrieve the clip. Markers that remain after surgery can later be used to target adjuvant radiation. It is recommended that new imaging be obtained prior to the initiation of adjuvant radiation. Given that radiotherapy is associated with clip migration, these images can serve as a crucial reference throughout the remainder of treatment.
The rate of negative margins after lumpectomy increases when biopsy markers are utilized in conjunction with WGL compared to WGL alone (13). Without a fiduciary marker, WGL must rely solely on prior imaging and, if present, characteristics of the target lesion. In addition to technical skill, there are many outside factors that can potentially hinder the success of WGL. Many of these factors center around characteristics inherent to the patient’s breast tissue or that of the target lesion itself. Both thin and large breasts create unique challenges. While breasts with abundant tissue can prove difficult to maneuver into the correct plane of view, imaging quality is often poor with particularly small or thin breasts due to inadequate filling of tissue into the compression paddle (14). Breasts that are very dense may produce distorted views. Characteristics of the disease itself can also hinder WGL, as subareolar lesions along with those that are very superficial or deep within the breast can prove difficult to successfully locate. Some of the recommended techniques for overcoming such difficult localizations include meticulous review of the patient’s prior imaging, changing the patient’s positioning, and utilizing alternative imaging modalities (15).
In our patient’s case, a fiduciary clip had been placed at the time of biopsy. On post-biopsy imaging, the clip was clearly visible and confirmed in the correct position. Despite this, the clip could not be adequately visualized to complete preoperative WGL on the day of surgery. Scenarios such as this are not uncommon in breast surgery and create significant challenges to physicians and patients alike. As it was in this case, often times there are no other landmarks to help identify the target lesion as all radiographic evidence is removed during preoperative biopsies. With no guidance, performing a lumpectomy then becomes akin to “finding a needle in a haystack”. A lumpectomy can still be accomplished under such conditions, however, it can be argued that the relative success, or lack thereof, defeats the purpose to the operation itself—to salvage as much healthy native breast tissue as possible.
In this case, several techniques were utilized to overcome the dilemma of a missing clip and complete a successful lumpectomy despite a lack of WGL. By correlating the patient’s prior imaging with the partial clip identification on the day of surgery, the skin was marked to serve as an intra-operative guide. While this appeared an elegant approach initially, we do not believe it served to benefit our attempts to locate the clip during surgery. We suspect this technique failed due to discrepancies between the patient’s prior imaging and the imaging obtained during attempted WGL. A combination of clip migration as well as variations in conditions such as positioning and equipment likely skewed our triangulation of the clip on the day of surgery. Contrarily, we feel the approach that was most helpful was intra-operative fluoroscopy. This technique was pivotal to finding the marking clip and allowing for completion of a successful oncologic operation while avoiding unnecessary injury to healthy breast tissue. It should be noted that this improvised technique was begotten through intra-operative consultation with the radiologist, thus further emphasizing the benefits of multidisciplinary collaboration in the care of patients with breast disease. Most importantly, in the end our patient was satisfied that she had received an appropriate oncologic surgery with no sacrifice to cosmesis.
In conclusion, the management of low-stage breast lesions has improved significantly over the last several decades with BCT becoming the standard of care. Successful implementation of BCT requires extensive radiographic documentation and close collaboration among multidisciplinary teams. As challenges can and will present themselves, clinicians must be aware of the potential pitfalls involving BCT so that they can be prepared to respond accordingly. In the event that WGL cannot be carried out on the day of surgery, techniques that may prove effective in overcoming this obstacle include multidisciplinary discussion, scrutinous review of all prior imaging studies, and the use of intra-operative fluoroscopy to visualize the location of the clip intra-operatively in real-time.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-112
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-112). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). This work was approved by the Institutional Review Board of HCA Graduate Medical Education, West Florida Division. Written informed consent was obtained from the patient presented.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312:665-73. [Crossref] [PubMed]
- Lazovich D, Solomon CC, Thomas DB, et al. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer 1999;86:628-37. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- El-Tamer MB, Ward BM, Schifftner T, et al. Morbidity and mortality following breast cancer surgery in women: national benchmarks for standards of care. Ann Surg 2007;245:665-71. [Crossref] [PubMed]
- Bruening W, Fontanarosa J, Tipton K, et al. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med 2010;152:238-46. [Crossref] [PubMed]
- White RR, Halperin TJ, Olson JA Jr, et al. Impact of core-needle breast biopsy on the surgical management of mammographic abnormalities. Ann Surg 2001;233:769-77. [Crossref] [PubMed]
- Srour MK, Kim S, Amersi F, et al. Comparison of wire localization, radioactive seed, and Savi scout® radar for management of surgical breast disease. Breast J 2020;26:406-13. [Crossref] [PubMed]
- Cheang E, Ha R, Thornton CM, et al. Innovations in image-guided preoperative breast lesion localization. Br J Radiol 2018;91:20170740 [Crossref] [PubMed]
- Teichgraeber DC, Martaindale S, Omofoye TS, et al. Immediate migration of biopsy clip markers after upright digital breast tomosynthesis-guided vacuum-assisted core biopsy. Acad Radiol 2020;27:204-9. [Crossref] [PubMed]
- Kass R, Kumar G, Klimberg VS, et al. Clip migration in stereotactic biopsy. Am J Surg 2002;184:325-31. [Crossref] [PubMed]
- Kalambo M, Dogan BE, Whitman GJ. Step by step: planning a needle localization procedure. Clin Imaging 2020;60:100-8. [Crossref] [PubMed]
- Pinkney DM, Mychajlowycz M, Shah BA. A prospective comparative study to evaluate the displacement of four commercially available breast biopsy markers. Br J Radiol 2016;89:20160149 [Crossref] [PubMed]
- Corsi F, Sorrentino L, Bossi D, et al. Preoperative localization and surgical margins in conservative breast surgery. Int J Surg Oncol 2013;2013:793819 [Crossref] [PubMed]
- Huang ML, Adrada BE, Candelaria R, et al. Stereotactic breast biopsy: pitfalls and pearls. Tech Vasc Interv Radiol 2014;17:32-9. [Crossref] [PubMed]
- Chesebro AL, Chikarmane SA, Ritner JA, et al. Troubleshooting to overcome technical challenges in image-guided breast biopsy. Radiographics 2017;37:705-18. [Crossref] [PubMed]
Cite this article as: Johnson D, Higginbotham M, Appiah L, Fan J, Misra S. Techniques for overcoming a missing clip during pre-operative needle localization for lumpectomy: case report. Ann Breast Surg 2021;5:10.


