
Contemporary management of atypical breast lesions identified on percutaneous biopsy: a narrative review
Introduction
Percutaneous biopsy is commonly performed to evaluate a mammographic abnormality, resulting in more than 1 million benign breast biopsies performed annually in the United States (1). Benign breast biopsies that have histologic epithelial abnormalities associated with increased risk of breast cancer are characterized as high-risk lesions or atypia, which represents >10% of all benign breast biopsies (2,3). The finding of a high-risk breast lesion on pathology from a percutaneous biopsy may be from under-sampling by core needle biopsy (CNB) with malignancy identified at the time of surgical excision. Alternatively, in the absence of malignancy upon excision, it represents an increased future risk of breast cancer (2). Continued improvement in the quality of breast cancer screening modalities necessitates a better understanding of the implications of these pathologic findings and development of appropriate management strategies that includes selection criteria for surgical excision, high risk screening, chemoprevention for risk reduction, and a more accurate assessment of future risk of breast cancer.
In this review, we will tackle the topic of atypical lesions of the breast identified on percutaneous biopsy. There is no clear consensus for who benefits from excision and who may be considered for observation. We will look into the unique aspects of each entity and how that may determine the benefit of excision. The question of how this diagnosis of atypia affects future breast cancer lifetime risk will also be addressed. Finally, potential interventions to mitigate future breast cancer will be discussed.
Flat epithelial atypia (FEA)
FEA was defined in 2003 by the World Health Organization (WHO) as a presumably neoplastic intraductal alteration characterized by enlarged acini and terminal ducts lined by layer(s) of monotonous epithelial cells with low-grade cytologic atypia, but lacking architectural atypia required for the diagnosis of atypical hyperplasia (AH) (4,5). FEA was thought to be a precursor lesion in the pathway for the development of breast cancer based on molecular data (6,7), and as it often occurs simultaneously with other high-risk lesions such as atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), and lobular carcinoma in situ (LCIS) (8). FEA is identified in anywhere from 0.7–12.2% of benign breast biopsies, usually presenting as screen-detected calcifications on mammogram (4,9-12). There is no current consensus on whether FEA identified on CNB without the presence of additional high-risk lesions warrants surgical excision (13).
When CNB identifies FEA, National Comprehensive Cancer Center (NCCN) recommendations suggest select patients may be suitable for monitoring in the absence of surgical excision, but does not provide guidance as to patient specifics (14). Historically, there has been a wide range in upgrade rate to underlying malignancy from 0–42% (4,10-12,15-30). In a meta-analysis of studies evaluating pure FEA on CNB by Rudin and colleagues, there was significant heterogeneity across studies; however, when restricting to higher-quality studies with stricter criteria, upgrade to malignancy was 7.5% (13). For those that did not have an underlying malignancy, excision for pure FEA identified ADH in 18.6% of surgical specimens. While not a malignancy itself, ADH carries an increased risk for future malignancy and may warrant more frequent screening and discussion of chemoprevention with the benefits of risk reduction discussed below, the information of which may be useful for the patient and her provider. Overall, the excision of pure FEA upgrades to malignancy or higher-risk atypia in up to 25% of patients, which may alter treatment recommendations. Therefore, the general recommendation would be that patients be considered for excision while also taking into account preferences and patient co-morbidities (13). However, more recent studies citing lower malignancy upgrade rates of <3% describe better lesion sampling with larger gauge needles (9–11 G), vacuum-assistance, and radiographic-pathologic concordance (31-38). This rate is on par with the Breast Imaging-Reporting and Data System (BIRADS) 3 lesions, which are typically recommended for surveillance over excision (39). With such a low rate of upgrade in contemporary studies (Table 1), observation over excision should be favored in most cases.
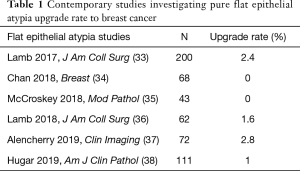
Full table
Future risk of malignancy does not appear to be significantly affected by the presence of FEA. In Said and colleagues’ study of 282 women with FEA with a median follow-up of 17 years, nearly half of women had coexisting AH with FEA. When comparing the future risk of breast cancer for those with FEA alone to those with FEA+AH, the risk with FEA alone was comparable with women with proliferative breast disease, and those with FEA+AH had elevated risk similar to women with AH alone (5). As the presence of FEA does not appear to be an independent risk factor for future breast cancer beyond that of any proliferative lesion, increased surveillance and chemoprevention may not be necessary.
Lobular neoplasia
The term “lobular neoplasia” (LN) was coined in 1978 by Haagensen to include both ALH and LCIS (40). LN account for approximately 3% of all breast biopsies (41-43), and are defined by monomorphic epithelial cell populations with minimal nuclear atypia that lacks cellular cohesion and contains frequent intracytoplasmic vacuoles, negative for E-cadherin because of somatic alterations of the CDH1 gene on the long arm of chromosome 16 (44-46). ALH and classic type LCIS are subclassifications of LN based on the quantity of atypia, with ALH defined as filling and distention of <50% of the acini in the terminal duct lobular unit and LCIS as >50% (47-49). LN is associated with underlying malignancy at the time of diagnosis on CNB, with upgrade rates ranging from 0–67%, as well as an elevated risk of future malignancy (50).
ALH
Page and colleagues established criteria for the diagnosis of ALH as a distinct entity in 1985 (45), which is frequently identified as an incidental finding on CNB (24,51). There is large variability in the studies attempting to define upgrade rate, many with small sample size, and ALH is often being grouped with LCIS (50). Many of these studies also did not exclude additional high-risk lesions such as intraductal papilloma, radial sclerosing lesion, or discordant lesions, which would presumably contribute to the higher upgrade rate. In contemporary studies that have controlled for all of those factors, the upgrade rate is <3% (36,41-43,52-56) (Table 2). Because of the low incidence of malignancy at a site of pure ALH, routine surgical excision is not recommended for ALH as an incidental finding with concordance between radiology and pathology (14,55,57,58).
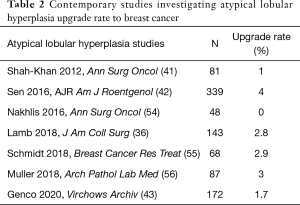
Full table
There are many factors that affect breast cancer risk, including lifetime estrogen exposure (age at menarche and menopause, parity, breastfeeding, hormone replacement therapy use), family history genetics, chest wall radiation at a young age, obesity, alcohol consumption, physical activity, in addition to breast specific features such as mammographic density and benign breast disease. Multiple large cohort studies have been performed investigating benign biopsies as a source of future risk [Nashville Breast Cohort, Partners Cohort, Nurses’ Health Study, Breast Cancer Surveillance Consortium, and Mayo Clinic Benign Breast Disease (BBD) Cohort]. Future breast cancer risk with a biopsy of ALH is 4-fold higher than the general population, which translates into an absolute risk of approximately 1–2%/year (50,59,60). Hartmann and colleagues using the Mayo BBD Cohort have gone even further by stratifying women by the number of atypical foci, demonstrating that increasing foci of atypia increases the future risk of breast cancer, with women ≥3 foci having a 25-year risk of malignancy close to 50% (50).
LCIS
Foote and Stewart first described LCIS in 1941 (61). LCIS, as with ALH, is typically an incidental finding on percutaneous biopsy performed for another imaging abnormality, but may occasionally be associated with microcalcifications on screening mammogram (9,24,51,53,62). Reports in the literature of upgrade rate after surgical excision for LCIS have been 15–33% (9,36,42,54).
It is important to recognize that there are several histologic subtypes of LCIS, the most common being classic type, which typically has an incidental presentation. Additional subtypes such as pleomorphic LCIS and LCIS with comedo-necrosis are typically associated with calcifications and may even be misclassified as DCIS. Less is known about these non-classic variants as most reports are single institution with small numbers, but reported upgrade rates to underlying malignancy are up to 70% (63-71). Therefore, surgical excision should be discussed for all non-classic type LCIS (24,66,72-74). However, more contemporary studies of classic type LCIS with radiographic-pathologic correlation has an upgrade rate of only 1–3% (24,55). Therefore, observation without excision should be considered for this entity (58). To further illustrate the low risk of upgrade upon excision of LCIS, Taylor and colleagues used the National Cancer Database (NCDB) to investigate the surgical practices for patients with LCIS. Nearly 85% of the 30,105 women with LCIS underwent excision, including 4% with unilateral mastectomy and 5.1% with bilateral mastectomy, representing a portion of women undergoing overtreatment for this entity (75).
Women with LCIS have an 8-fold relative risk of developing a future breast cancer, translating into an absolute risk of approximately 2%/year (51). Other contributing factors are age at diagnosis and number of atypical foci (58). Based on a large SEER study, it appears that race and ethnicity also play a role in future breast cancer risk, with Black women with LCIS having a 1.3× higher risk of future breast cancer than white women with LCIS (76). It is important that risk stratification be performed considering these factors allowing high-risk screening and offering chemoprevention to be considered when appropriate.
ADH
ADH, like DCIS, frequently presents as calcifications on screening mammogram and is identified in 8–17% of breast biopsies (31,53,77). Unfortunately, ADH and DCIS are histologically virtually identical, with the distinction being quantity of atypia and nuclear grade. A lesion is characterized as ADH if low-grade cytologic atypia and monomorphism combined with epithelial architectural complexity is involving less than two contiguous membrane-bound spaces and measures less than 2 mm in linear extent (51). Because the amount of sampling of the lesion can be the distinction between ADH and DCIS, surgical excision is typically recommended for ADH to rule out an underlying malignancy (14,53,78). An additional difficulty with ADH is the interobserver variability between pathologists to distinguish ADH from DCIS or usual ductal hyperplasia, as these diagnoses are made on morphology alone, without the aid of specific immunohistochemical stains. One study cites a concordance rate between pathologists for atypia to be as low as 48% (79). Clinicians and patients should not hesitate to seek a second opinion or at least request another pathology review before proceeding with excision or surveillance under these circumstances to confirm the diagnosis.
Recent studies report an overall upgrade rate of ADH to malignancy of 15–25% and approximately 3% risk of invasive breast cancer alone (3,9,36,62,77,80-92) (Table 3). This then translates to 75–85% not having any malignancy and nearly 97% not having invasive breast cancer. This has resulted in many studies trying to identify those patients at the lowest risk of having a current malignancy, which could be successfully managed with observation over surgical excision.
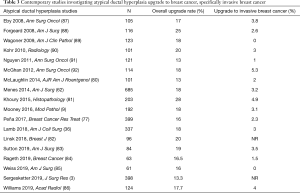
Full table
The earliest study investigating the feasibility of selecting a low-risk for upgrade group for observation incorporated patient factors, including younger age (<50 years), screen-detected lesions (no palpable masses or nipple discharge), radiographic features such as small lesion size (<1.5 cm), imaging presentation of calcifications without a mass, and pathologic features including low burden of atypical foci (only 1 focus). When patients meeting those restrictions underwent excision, this resulted in an upgrade rate of 5.6% (93). Subsequent studies identified their lowest-risk populations to consider for observation to be those with radiographic calcifications without mass, and also incorporating pathologic features of no individual cell necrosis and the extent of the radiographic lesion removal by biopsy (>95%). This low-risk group had an upgrade rate of 6% (91). This is further refined to allow observation when there is no necrosis, and using a combination of volume of atypia with the degree of sampling (only 1 focus and >50% removed or ≤3 foci and >90% removed), with a resulting upgrade rate of 4.9% (77). In general, the lowest risk for upgrade is in concordant lesions without a mass, small lesions, and well-sampled lesions with complete or near complete removal (32). When these criteria are not met, surgical excision should be performed (78,94,95).
As mentioned above, if ADH does upgrade to malignancy, most upgrades are to DCIS. Outside of a clinical trial, the standard of care for the management of DCIS is surgical excision followed by whole breast irradiation after lumpectomy and endocrine therapy. However, the standard management of DCIS is being called into question, with several international trials currently accruing offering observation over excision for grade 1–2 hormone-positive DCIS. Thus, the routine surgical excision of ADH should also be questioned. If the site of ADH is not excised, patients do not appear to be at increased risk of malignancy specifically at that location. Menen and colleagues demonstrated patients with ADH meeting the above low-risk criteria can be safely offered observation over excision, with the subsequent breast cancer rate of 5.6% over a median three year follow up and the majority not being at the ADH biopsy site (96).
Whether or not the ADH site is excised, future breast cancer risk persists and is a global risk, not isolated to the site of ADH diagnosis, with 40% of diagnoses being contralateral (50,59,60,62). Similar to ALH, having a diagnosis of ADH carries a four-fold risk of future breast cancer, translating into 1–2%/year absolute risk, with no plateau seen with extensive follow up (60,97).
High risk screening and chemoprevention
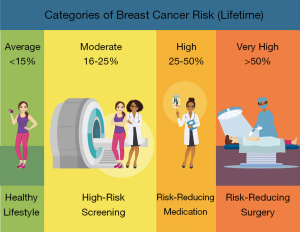
For all women who have LN or ADH, future breast cancer risk is elevated to varying degrees. NCCN management recommendations for patients diagnosed with atypia include clinical encounter every six to twelve months, annual MMG after age 30, consider annual MRI after age 25, risk reduction strategies, and breast awareness (Figure 1) (14). This recommendation is regardless of if the site of atypia undergoes excision. American Society of Clinical Oncology (ASCO) guidelines recommend chemoprevention be discussed with women with a 5-year absolute risk of breast cancer of 1.7% or higher (98,99). Chemoprevention with tamoxifen, raloxifene, anastrozole, or exemestane results in a 50% reduction in future malignancy risk (100-102). This risk reduction is even more significant in women with LN and ADH ranging from 65–70%, when compared to those with a family history of breast cancer without an atypia diagnosis (50,59).
Chemoprevention acceptance has been historically low even for the treatment of DCIS, let alone for women with a family history or history of LH/ADH (103-106). Reasons for this low rate include clinicians and women underestimating their future risk of breast cancer, as well as women’s fear of adverse effects from this therapy. Tamoxifen is associated with elevated rates of endometrial cancer (RR 2.25), cataracts (RR 1.22), and thromboembolic events (RR 1.93), while anastrozole and exemestane are associated with bone loss. Future directions for prevention include low dose tamoxifen of 5 mg daily have much fewer side effects compared to the traditional dosage of 20 mg daily with a 52% reduction of ipsilateral breast cancer events, and a 76% reduction in contralateral breast cancer events (107). Because the systemic side effects of oral medication are a large reason for the lack of adherence or acceptance for chemoprevention, a topical formulation of tamoxifen (4-OHT) that can be applied to breast skin is currently under investigation (108,109).
Conclusions
Many women present with screen detected calcifications that undergo percutaneous biopsy that results in a diagnosis of atypia. However, the presence of atypia on CNB should no longer be an automatic indication for surgical excision. Emphasis should be placed on assessing radiographic and pathologic concordance, and further consideration for observation over excision for well-sampled, concordant lesions, particularly for FEA and LN. Surgical excision should be reserved for discordant lesions, large lesions that are under-sampled, and for ADH in many cases. Recognition that future breast cancer risk persists even after atypia excision is vital, and appropriate counseling about lifestyle modification, increased intensity of breast screening, and discussion of chemoprevention should be offered.
Acknowledgments
Holly Zink, MSA, ACRP-CP, Medical Writer for the Department of Surgery at the University of Kansas Health System designed Figure 1 and assisted in formatting and editing this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Katharine Yao) for the series “A Practical Guide to Management of Benign Breast Disease” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/abs-20-117). The series “A Practical Guide to Management of Benign Breast Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gutwein LG, Ang DN, Liu H, et al. Utilization of minimally invasive breast biopsy for the evaluation of suspicious breast lesions. Am J Surg 2011;202:127-32. [Crossref] [PubMed]
- Racz JM, Carter JM, Degnim AC. Challenging Atypical Breast Lesions Including Flat Epithelial Atypia, Radial Scar, and Intraductal Papilloma. Ann Surg Oncol 2017;24:2842-7. [Crossref] [PubMed]
- Sergesketter AR, Thomas SM, Fayanju OM, et al. The Influence of Age on the Histopathology and Prognosis of Atypical Breast Lesions. J Surg Res 2019;241:188-98. [Crossref] [PubMed]
- Martel M, Barron-Rodriguez P, Tolgay Ocal I, et al. Flat DIN 1 (flat epithelial atypia) on core needle biopsy: 63 Cases identified retrospectively among 1,751 core biopsies performed over an 8-year period (1992-1999). Virchows Arch 2007;451:883-91. [Crossref] [PubMed]
- Said SM, Visscher DW, Nassar A, et al. Flat epithelial atypia and risk of breast cancer: A Mayo cohort study. Cancer 2015;121:1548-55. [Crossref] [PubMed]
- Reis-Filho JS, Simpson PT, Gale T, et al. The molecular genetics of breast cancer: The contribution of comparative genomic hybridization. Pathol Res Pract 2005;201:713-25. [Crossref] [PubMed]
- Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J Pathol 2011;223:307-17. [Crossref] [PubMed]
- Schnitt SJ, Vincent-Salomon A. Columnar Cell Lesions of the Breast. Adv Anat Pathol 2003;10:113-24. [Crossref] [PubMed]
- Mooney KL, Bassett LW, Apple SK. Upgrade rates of high-risk breast lesions diagnosed on core needle biopsy: A single-institution experience and literature review. Mod Pathol 2016;29:1471-84. [Crossref] [PubMed]
- Becker AK, Gordon PB, Harrison DA, et al. Flat Ductal Intraepithelial Neoplasia 1A Diagnosed at Stereotactic Core Needle Biopsy: Is Excisional Biopsy Indicated? AJR Am J Roentgenol 2013;200:682-8. [Crossref] [PubMed]
- Kunju LP, Kleer CG. Significance of flat epithelial atypia on mammotome core needle biopsy: Should it be excised? Hum Pathol 2007;38:35-41. [Crossref] [PubMed]
- Piubello Q, Parisi A, Eccher A, et al. Flat Epithelial Atypia on Core Needle Biopsy: Which is the Right Management? Am J Surg Pathol 2009;33:1078-84. [Crossref] [PubMed]
- Rudin AV, Hoskin TL, Fahy A, et al. Flat Epithelial Atypia on Core Biopsy and Upgrade to Cancer: A Systematic Review and Meta-Analysis. Ann Surg Oncol 2017;24:3549-58. [Crossref] [PubMed]
- National Comprehensive Cancer Network Clinical Guidelines [Internet]. Breast Cancer Screening and Diagnosis, v1 2019 [cited 2020 May 9]. p. BSCR-3,8,9. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf
- Lavoué V, Roger CM, Poilblanc M, et al. Pure flat epithelial atypia (DIN 1a) on core needle biopsy: Study of 60 biopsies with follow-up surgical excision. Breast Cancer Res Treat 2011;125:121-6. [Crossref] [PubMed]
- Peres A, Barranger E, Becette V, et al. Rates of upgrade to malignancy for 271 cases of flat epithelial atypia (FEA) diagnosed by breast core biopsy. Breast Cancer Res Treat 2012;133:659-66. [Crossref] [PubMed]
- Uzoaru I, Morgan BR, Liu ZG, et al. Flat epithelial atypia with and without atypical ductal hyperplasia: to re-excise or not. Results of a 5-year prospective study. Virchows Arch 2012;461:419-23. [Crossref] [PubMed]
- Bianchi S, Bendinelli B, Castellano I, et al. Morphological parameters of flat epithelial atypia (FEA) in stereotactic vacuum-assisted needle core biopsies do not predict the presence of malignancy on subsequent surgical excision. Virchows Arch 2012;461:405-17. [Crossref] [PubMed]
- Yamaguchi R, Tanaka M, Tse GM, et al. Pure flat epithelial atypia is uncommon in subsequent breast excisions for atypical epithelial proliferation. Cancer Sci 2012;103:1580-5. [Crossref] [PubMed]
- Polom K, Murawa D, Murawa P. Flat epithelial atypia diagnosed on core needle biopsy—Clinical challenge. Rep Pract Oncol Radiother 2012;17:93-6. [Crossref] [PubMed]
- Khoumais NA, Scaranelo AM, Moshonov H, et al. Incidence of Breast Cancer in Patients with Pure Flat Epithelial Atypia Diagnosed at Core-Needle Biopsy of the Breast. Ann Surg Oncol 2013;20:133-8. [Crossref] [PubMed]
- Villa A, Chiesa F, Massa T, et al. Flat Epithelial Atypia: Comparison Between 9-Gauge and 11-Gauge Devices. Clin Breast Cancer 2013;13:450-4. [Crossref] [PubMed]
- Ceugnart L, Doualliez V, Chauvet MP, et al. Pure flat epithelial atypia: Is there a place for routine surgery? Diagn Interv Imaging 2013;94:861-9. [Crossref] [PubMed]
- Calhoun BC. Core Needle Biopsy of the Breast: An Evaluation of Contemporary Data. Surg Pathol Clin 2018;11:1-16. [Crossref] [PubMed]
- Uzan C, Mazouni C, Ferchiou M, et al. A model to predict the risk of upgrade to malignancy at surgery in atypical breast lesions discovered on percutaneous biopsy specimens. Ann Surg Oncol 2013;20:2850-7. [Crossref] [PubMed]
- Chivukula M, Bhargava R, Tseng G, et al. Clinicopathologic Implications of “Flat Epithelial Atypia” in Core Needle Biopsy Specimens of the Breast. Am J Clin Pathol 2009;131:802-8. [Crossref] [PubMed]
- Tomasino RM, Morello V, Gullo A, et al. Assessment of “grading” with Ki-67 and c-kit immunohistochemical expressions may be a helpful tool in management of patients with flat epithelial atypia (FEA) and columnar cell lesions (CCLs) on core breast biopsy. J Cell Physiol 2009;221:343-9. [Crossref] [PubMed]
- Lee TYJ, MacIntosh RF, Rayson D, et al. Flat Epithelial Atypia on Breast Needle Core Biopsy: A Retrospective Study with Clinical-Pathological Correlation. Breast J 2010;16:377-83. [Crossref] [PubMed]
- Flegg KM, Flaherty JJ, Bicknell AM, et al. Surgical outcomes of borderline breast lesions detected by needle biopsy in a breast screening program. World J Surg Oncol 2010;8:78. [Crossref] [PubMed]
- Sohn V, Porta R, Brown T. Flat Epithelial Atypia of the Breast on Core Needle Biopsy: An Indication for Surgical Excision. Mil Med 2011;176:1347-50. [Crossref] [PubMed]
- Collins LC. Precursor Lesions of the Low-Grade Breast Neoplasia Pathway. Surg Pathol Clin 2018;11:177-97. [Crossref] [PubMed]
- Schiaffino S, Gristina L, Villa A, et al. Flat epithelial atypia: conservative management of patients without residual microcalcifications post-vacuum-assisted breast biopsy. Br J Radiol 2018;91:20170484 [Crossref] [PubMed]
- Lamb LR, Bahl M, Gadd MA, et al. Flat Epithelial Atypia: Upgrade Rates and Risk-Stratification Approach to Support Informed Decision Making. J Am Coll Surg 2017;225:696-701. [Crossref] [PubMed]
- Chan PMY, Chotai N, Lai ES, et al. Majority of flat epithelial atypia diagnosed on biopsy do not require surgical excision. Breast 2018;37:13-7. [Crossref] [PubMed]
- McCroskey Z, Sneige N, Herman CR, et al. Flat epithelial atypia in directional vacuum-assisted biopsy of breast microcalcifications: surgical excision may not be necessary. Mod Pathol 2018;31:1097-106. [Crossref] [PubMed]
- Lamb LR, Bahl M, Hughes KS, et al. Pathologic Upgrade Rates of High-Risk Breast Lesions on Digital Two-Dimensional vs Tomosynthesis Mammography. J Am Coll Surg 2018;226:858-67. [Crossref] [PubMed]
- Alencherry E, Goel R, Gore S, et al. Clinical, imaging, and intervention factors associated with the upgrade of isolated flat epithelial atypia. Clin Imaging 2019;54:21-4. [Crossref] [PubMed]
- Hugar SB, Bhargava R, Dabbs DJ, et al. Isolated Flat Epithelial Atypia on Core Biopsy Specimens Is Associated With a Low Risk of Upgrade at Excision. Am J Clin Pathol 2019;151:511-5. [Crossref] [PubMed]
- Baum JK, Hanna LG, Acharyya S, et al. Use of BI-RADS 3-Probably Benign Category in the American College of Radiology Imaging Network Digital Mammographic Imaging Screening Trial. Radiology 2011;260:61-7. [Crossref] [PubMed]
- Haagensen CD, Lane N, Lattes R, et al. Lobular neoplasia (so-called lobular carcinoma in situ) of the breast. Cancer 1978;42:737. [Crossref] [PubMed]
- Shah-Khan MG, Geiger XJ, Reynolds C, et al. Long-Term Follow-up of Lobular Neoplasia (Atypical Lobular Hyperplasia/Lobular Carcinoma In Situ) Diagnosed on Core Needle Biopsy. Ann Surg Oncol 2012;19:3131-8. [Crossref] [PubMed]
- Sen LQC, Berg WA, Hooley RJ, et al. Core Breast Biopsies Showing Lobular Carcinoma In Situ Should Be Excised and Surveillance Is Reasonable for Atypical Lobular Hyperplasia. AJR Am J Roentgenol 2016;207:1132-45. [Crossref] [PubMed]
- Genco IS, Tugertimur B, Chang Q, et al. Outcomes of classic lobular neoplasia diagnosed on breast core needle biopsy: A retrospective multi-center study. Virchows Arch 2020;476:209-17. [Crossref] [PubMed]
- Wen HY, Brogi E. Lobular Carcinoma In Situ. Surg Pathol Clin 2018;11:123-45. [Crossref] [PubMed]
- Page DL, Dupont WD, Rogers LW, et al. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 1985;55:2698-708. [Crossref] [PubMed]
- Hwang H, Barke LD, Mendelson EB, et al. Atypical lobular hyperplasia and classic lobular carcinoma in situ in core biopsy specimens: routine excision is not necessary. Mod Pathol 2008;21:1208. [Crossref] [PubMed]
- Page DL, Kidd TE, Dupont WD, et al. Lobular neoplasia of the breast: Higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol 1991;22:1232-9. [Crossref] [PubMed]
- Tavassoli FA. Lobular neoplasia: evolution of its significance and morphologic spectrum. Int J Surg Pathol 2010;18:174S. [Crossref] [PubMed]
- Rosen PP, Kosloff C, Lieberman PH, et al. Lobular carcinoma in situ of the breast: detailed analysis of 99 patients with average follow-up of 24 years. Am J Surg Pathol 1978;2:225. [Crossref] [PubMed]
- Hartmann LC, Degnim AC, Santen RJ, et al. Atypical Hyperplasia of the Breast--Risk Assessment and Management Options. N Engl J Med 2015;372:78-89. [Crossref] [PubMed]
- Racz JM, Carter JM, Degnim AC. Lobular Neoplasia and Atypical Ductal Hyperplasia on Core Biopsy: Current Surgical Management Recommendations. Ann Surg Oncol 2017;24:2848-54. [Crossref] [PubMed]
- Allen S, Levine EA, Lesko N, et al. Is Excisional Biopsy and Chemoprevention Warranted in Patients With Atypical Lobular Hyperplasia on Core Biopsy? Am Surg 2015;81:876-8. [Crossref] [PubMed]
- Lewin AA, Mercado CL. Atypical Ductal Hyperplasia and Lobular Neoplasia: Update and Easing of Guidelines. AJR Am J Roentgenol 2020;214:265-75. [Crossref] [PubMed]
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of Adjacent Synchronous Invasive Carcinoma and/or Ductal Carcinoma In-situ in Patients with Lobular Neoplasia on Core Biopsy: Results from a Prospective Multi-Institutional Registry (TBCRC 020). Ann Surg Oncol 2016;23:722-8. [Crossref] [PubMed]
- Schmidt H, Arditi B, Wooster M, et al. Observation versus excision of lobular neoplasia on core needle biopsy of the breast. Breast Cancer Res Treat 2018;168:649-54. [Crossref] [PubMed]
- Muller KE, Roberts E, Zhao L, et al. Isolated Atypical Lobular Hyperplasia Diagnosed on Breast Biopsy: Low Upgrade Rate on Subsequent Excision With Long-Term Follow-up. Arch Pathol Lab Med 2018;142:391-5. [Crossref] [PubMed]
- Nakhlis F. How Do We Approach Benign Proliferative Lesions? Curr Oncol Rep 2018;20:34. [Crossref] [PubMed]
- Thomas PS. Diagnosis and Management of High-Risk Breast Lesions. J Natl Compr Canc Netw 2018;16:1391-6. [Crossref] [PubMed]
- Coopey SB, Mazzola E, Buckley JM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat 2012;136:627-33. [Crossref] [PubMed]
- Donaldson AR, McCarthy C, Goraya S, et al. Breast cancer risk associated with atypical hyperplasia and lobular carcinoma in situ initially diagnosed on core-needle biopsy. Cancer 2018;124:459-65. [Crossref] [PubMed]
- Foote FW, Stewart FW. Lobular carcinoma in situ: A rare form of mammary cancer. Am J Pathol 1941;17:491-6. [PubMed]
- Menes TS, Rosenberg R, Balch S, et al. Upgrade of high-risk breast lesions detected on mammography in the Breast Cancer Surveillance Consortium. Am J Surg 2014;207:24-31. [Crossref] [PubMed]
- Wazir U, Wazir A, Wells C, et al. Pleomorphic lobular carcinoma in situ: Current evidence and a systemic review. Oncol Lett 2016;12:4863-8. [Crossref] [PubMed]
- Singh K, Paquette C, Kalife ET, et al. Evaluating agreement, histological features, and relevance of separating pleomorphic and florid lobular carcinoma in situ subtypes. Hum Pathol 2018;78:163-70. [Crossref] [PubMed]
- Shamir ER, Chen YY, Chu T, et al. Pleomorphic and Florid Lobular Carcinoma In Situ Variants of the Breast: A Clinicopathologic Study of 85 Cases With and Without Invasive Carcinoma From a Single Academic Center. Am J Surg Pathol 2019;43:399-408. [Crossref] [PubMed]
- Masannat YA, Husain E, Roylance R, et al. Pleomorphic LCIS what do we know? A UK multicenter audit of pleomorphic lobular carcinoma in situ. Breast 2018;38:120-4. [Crossref] [PubMed]
- Hoffman DI, Zhang PJ, Tchou J. Breast-conserving surgery for pure non-classic lobular carcinoma in situ: A single institution’s experience. Surg Oncol 2019;28:190-4. [Crossref] [PubMed]
- Desai AA, Jimenez RE, Hoskin TL, et al. Treatment Outcomes for Pleomorphic Lobular Carcinoma In Situ of the Breast. Ann Surg Oncol 2018;25:3064-8. [Crossref] [PubMed]
- Savage JL, Jeffries DO, Noroozian M, et al. Pleomorphic Lobular Carcinoma In Situ: Imaging Features, Upgrade Rate, and Clinical Outcomes. AJR Am J Roentgenol 2018;211:462-7. [Crossref] [PubMed]
- Guo T, Wang Y, Shapiro N, et al. Pleomorphic Lobular Carcinoma in Situ Diagnosed by Breast Core Biopsy: Clinicopathologic Features and Correlation With Subsequent Excision. Clin Breast Cancer 2018;18:e449-54. [Crossref] [PubMed]
- Fasola CE, Chen JJ, Jensen KC, et al. Characteristics and clinical outcomes of pleomorphic lobular carcinoma in situ of the breast. Breast J 2018;24:66-9. [Crossref] [PubMed]
- Chivukula M, Haynik DM, Brufsky A, et al. Pleomorphic Lobular Carcinoma In Situ (PLCIS) on Breast Core Needle Biopsies: Clinical Significance and Immunoprofile. Am J Surg Pathol 2008;32:1721-6. [Crossref] [PubMed]
- Flanagan MR, Rendi MH, Calhoun KE, et al. Pleomorphic Lobular Carcinoma In Situ: Radiologic-Pathologic Features and Clinical Management. Ann Surg Oncol 2015;22:4263-9. [Crossref] [PubMed]
- Bagaria SP, Shamonki J, Kinnaird M, et al. The Florid Subtype of Lobular Carcinoma In Situ: Marker or Precursor for Invasive Lobular Carcinoma? Ann Surg Oncol 2011;18:1845-51. [Crossref] [PubMed]
- Taylor LJ, Steiman J, Schumacher JR, et al. Surgical Management of Lobular Carcinoma In Situ: Analysis of the National Cancer Database. Ann Surg Oncol 2018;25:2229-34. [Crossref] [PubMed]
- Dania V, Liu Y, Ademuyiwa F, et al. Associations of race and ethnicity with risk of developing invasive breast cancer after lobular carcinoma in situ. Breast Cancer Res 2019;21:120. [Crossref] [PubMed]
- Peña A, Shah SS, Fazzio RT, et al. Multivariate model to identify women at low risk of cancer upgrade after a core needle biopsy diagnosis of atypical ductal hyperplasia. Breast Cancer Res Treat 2017;164:295-304. [Crossref] [PubMed]
- Schiaffino S, Calabrese M, Melani EF, et al. Upgrade Rate of Percutaneously Diagnosed Pure Atypical Ductal Hyperplasia: Systematic Review and Meta-Analysis of 6458 Lesions. Radiology 2020;294:76-86. [Crossref] [PubMed]
- Elmore JG, Longton GM, Carney PA, et al. Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 2015;313:1122-32. [Crossref] [PubMed]
- McLaughlin CT, Neal CH, Helvie MA. Is the Upgrade Rate of Atypical Ductal Hyperplasia Diagnosed by Core Needle Biopsy of Calcifications Different for Digital and Film-Screen Mammography? AJR Am J Roentgenol 2014;203:917-22. [Crossref] [PubMed]
- Khoury T, Chen X, Wang D, et al. Nomogram to predict the likelihood of upgrade of atypical ductal hyperplasia diagnosed on a core needle biopsy in mammographically detected lesions. Histopathology 2015;67:106-20. [Crossref] [PubMed]
- Linsk A, Mehta TS, Dialani V, et al. Surgical upgrade rate of breast atypia to malignancy: An academic center’s experience and validation of a predictive model. Breast J 2018;24:115-9. [Crossref] [PubMed]
- Sutton T, Farinola M, Johnson N, et al. Atypical ductal hyperplasia: Clinicopathologic factors are not predictive of upgrade after excisional biopsy. Am J Surg 2019;217:848-50. [Crossref] [PubMed]
- Rageth CJ, Rubenov R, Bronz C, et al. Atypical ductal hyperplasia and the risk of underestimation: tissue sampling method, multifocality, and associated calcification significantly influence the diagnostic upgrade rate based on subsequent surgical specimens. Breast Cancer 2019;26:452-8. [Crossref] [PubMed]
- Weiss JB, Do WS, Forte DM, et al. Is bigger better? Twenty-year institutional experience of atypical ductal hyperplasia discovered by core needle biopsy. Am J Surg 2019;217:906-9. [Crossref] [PubMed]
- Williams KE, Amin AL, Hill J, et al. Radiologic and Pathologic Features Associated With Upgrade of Atypical Ductal Hyperplasia at Surgical Excision. Acad Radiol 2019;26:893-9. [Crossref] [PubMed]
- Eby PR, Ochsner JE, DeMartini WB, et al. Is Surgical Excision Necessary for Focal Atypical Ductal Hyperplasia Found at Stereotactic Vacuum-Assisted Breast Biopsy? Ann Surg Oncol 2008;15:3232-8. [Crossref] [PubMed]
- Forgeard C, Benchaib M, Guerin N, et al. Is surgical biopsy mandatory in case of atypical ductal hyperplasia on 11-gauge core needle biopsy? A retrospective study of 300 patients. Am J Surg 2008;196:339-45. [Crossref] [PubMed]
- Wagoner MJ, Laronga C, Acs G. Extent and histologic pattern of atypical ductal hyperplasia present on core needle biopsy specimens of the breast can predict ductal carcinoma in situ in subsequent excision. Am J Clin Pathol 2009;131:112-21. [Crossref] [PubMed]
- Kohr JR, Eby PR, Allison KH, et al. Risk of Upgrade of Atypical Ductal Hyperplasia after Stereotactic Breast Biopsy: Effects of Number of Foci and Complete Removal of Calcifications. Radiology 2010;255:723-30. [Crossref] [PubMed]
- Nguyen CV, Albarracin CT, Whitman GJ, et al. Atypical ductal hyperplasia in directional vacuum-assisted biopsy of breast microcalcifications: Considerations for surgical excision. Ann Surg Oncol 2011;18:752-61. [Crossref] [PubMed]
- McGhan LJ, Pockaj BA, Wasif N, et al. Atypical ductal hyperplasia on core biopsy: An automatic trigger for excisional biopsy? Ann Surg Oncol 2012;19:3264-9. [Crossref] [PubMed]
- Ko E, Han W, Lee JW, et al. Scoring system for predicting malignancy in patients diagnosed with atypical ductal hyperplasia at ultrasound-guided core needle biopsy. Breast Cancer Res Treat 2008;112:189-95. [Crossref] [PubMed]
- Racz JM, Degnim AC. When Does Atypical Ductal Hyperplasia Require Surgical Excision? Vol 27, Surgical Oncology Clinics of North America. W.B. Saunders; 2018:23-32.
- American Society of Breast Surgeons [Internet]. Consensus Guideline on Concordance Assessment of Image-Guided Breast Biopsies and Management of Borderline or High-Risk Lesions 2016 [cited 2020 May 9]. p. 1-12. Available online: http://www.breastsurgeons.org/statements/PDF_Statements/Concordance_Assessment.pdf
- Menen RS, Ganesan N, Bevers T, et al. Long-Term Safety of Observation in Selected Women Following Core Biopsy Diagnosis of Atypical Ductal Hyperplasia. Ann Surg Oncol 2017;24:70-6. [Crossref] [PubMed]
- King TA, Pilewskie M, Muhsen S, et al. Lobular Carcinoma in Situ: A 29-Year Longitudinal Experience Evaluating Clinicopathologic Features and Breast Cancer Risk. J Clin Oncol 2015;33:3945-52. [Crossref] [PubMed]
- Visvanathan K, Hurley P, Bantug E, et al. Use of Pharmacologic Interventions for Breast Cancer Risk Reduction: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2013;31:2942-62. [Crossref] [PubMed]
- Brewster AM, Thomas P, Brown P, et al. A System-Level Approach to Improve the Uptake of Antiestrogen Preventive Therapy among Women with Atypical Hyperplasia and Lobular Cancer. Cancer Prev Res (Phila) 2018;11:295. [Crossref] [PubMed]
- Cuzick J, Sestak I, Forbes JF, et al. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet 2020;395:117-22. [Crossref] [PubMed]
- Nelson HD, Fu R, Zakher B, et al. Medication Use for the Risk Reduction of Primary Breast Cancer in Women: A Systematic Review for the U.S. Preventive Services Task Force Risk-Reducing Medications for Breast Cancer ii Pacific Northwest EPC Acknowledgments [Internet]. Rockville (MD); 2019. Available online: www.ahrq.govwww.ohsu.edu/epc
- Owens DK, Davidson KW, Krist AH, et al. Medication Use to Reduce Risk of Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2019;322:857-67. [Crossref] [PubMed]
- Karavites LC, Kane AK, Zaveri S, et al. Tamoxifen Acceptance and Adherence among Patients with Ductal Carcinoma In Situ (DCIS) Treated in a Multidisciplinary Setting. Cancer Prev Res (Phila) 2017;10:389. [Crossref] [PubMed]
- Roche CA, Tang R, Coopey SB, et al. Chemoprevention acceptance and adherence in women with high-risk breast lesions. Breast J 2019;25:190-5. [Crossref] [PubMed]
- Roetzheim RG, Lee JH, Fulp W, et al. Acceptance and adherence to chemoprevention among women at increased risk of breast cancer. Breast 2015;24:51-6. [Crossref] [PubMed]
- Flanagan MR, Zabor EC, Stempel M, et al. Chemoprevention Uptake for Breast Cancer Risk Reduction Varies by Risk Factor. Ann Surg Oncol 2019;26:2127-35. [Crossref] [PubMed]
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J Clin Oncol 2019;37:1629-37. [Crossref] [PubMed]
- Lee O, Khan SA. Novel routes for administering chemoprevention: Local transdermal therapy to the breasts. Semin Oncol 2016;43:107-15. [Crossref] [PubMed]
- Lee O, Ivancic D, Allu S, et al. Local transdermal therapy to the breast for breast cancer prevention and DCIS therapy: preclinical and clinical evaluation. Cancer Chemother Pharmacol 2015;76:1235-46. [Crossref] [PubMed]
Cite this article as: Amin AL, Wagner JL. Contemporary management of atypical breast lesions identified on percutaneous biopsy: a narrative review. Ann Breast Surg 2021;5:9.

