
Diagnosis and management of phyllodes tumors of the breast
Introduction
Phyllodes tumors are fibroepithelial lesions of the breast representing less than 1% of all breast tumors (1). Histologically, they can resemble fibroadenomas and are differentiated by stromal hyper cellularity and a characteristic “leaf-like” architecture (2). Phyllodes tumors are classified as benign, borderline, or malignant based on histologic grading of tumor border, stromal cellularity, mitotic figures, the presence of atypia, and stromal overgrowth (3). Categorizing these tumors is somewhat subjective with variance in classification among pathologists. Phyllodes tumors often present in younger women and are often described as firm, palpable masses demonstrating rapid growth. The mainstay of treatment for phyllodes tumors is surgical excision with some debate on the appropriate margin. In the setting of malignant phyllodes tumors, the benefit of adjuvant therapy is not well established and is recommended on a case by case basis. In this paper we aim to present an overview of the diagnosis and management of benign as well as malignant phyllodes tumors.
Presentation and diagnosis
Phyllodes tumors are a rare subtype of fibroepithelial lesions which may be difficult to distinguish from fibroadenomas (4). Presentation is typically in women in their forties compared to younger women who are diagnosed with fibroadenomas (5). Phyllodes tumors are more common in Asian women, and may present earlier in age (6). Patients with a TP53 mutation (Li Fraumeni syndrome) have increased risk for developing phyllodes tumors (7). The work up of a phyllodes tumor includes mammography and ultrasound. Phyllodes tumors can be difficult to distinguish from fibroadenomas radiographically, although certain features such as larger size, hyper dense appearance on mammography, heterogeneous echo, the presence of round cysts within the mass and internal clefts on ultrasound may be suggestive, but not pathognomonic for phyllodes tumors (8). A core needle biopsy is necessary to aide in distinguishing fibroadenomas from phyllodes tumors but may still possess some uncertainty histologically due to overlapping features and tumor heterogeneity (9). In this instance, lesions may be classified as a fibroepithelial lesion with recommendations for complete surgical excision for definitive diagnosis.
The World Health Organization (WHO) criteria for classifying phyllodes tumors evaluates tumor border, stromal cellularity, stromal atypia, mitotic activity, and stromal overgrowth to categorize tumors as benign, borderline or malignant (3) (Table 1). Phyllodes tumors may present with features from more than one category making classification somewhat difficult and subjective.
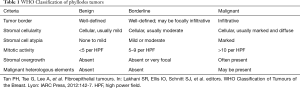
Full table
Benign phyllodes tumors (BPTs) are the most common (60–75%) are typically well defined with mild stromal cellularity, no to mild atypia, few mitoses, with no stromal overgrowth. Borderline phyllodes tumors may demonstrate moderate stromal cellularity, mild to moderate atypia and more frequent mitotic figures, 5–9 per high power field (HPF). Malignant phyllodes tumors demonstrate infiltrative borders, diffuse stromal cellularity, marked atypia and >10 mitoses per HPF. A phyllodes tumor presenting with malignant heterologous elements is sufficient for the diagnosis of a malignant phyllodes even in the absence of other malignant features (3).
Surgical management
Once a diagnosis of phyllodes tumor is confirmed or suspected, the mainstay of treatment is surgical excision. A mass with increased stromal cellularity suspicious for a phyllodes tumor should also be excised, though surgical excision may be more conservative with the understanding re-excision with wider margins may be necessary if final pathology is consistent with a phyllodes tumor. It is important to clearly denote the prior resection cavity with clips or identify the resection cavity with ultrasound in the event re-excision is necessary. Breast conservation is an appropriate treatment for phyllodes tumors if good aesthetic and oncologic outcomes are feasible (10). Despite the demonstrated equivalence with breast conservation, mastectomy is still performed in nearly 50% of cases due to large tumor size and recommendations for wide local excision (11). The majority of tumors larger than 5cm will undergo mastectomy though advanced oncoplastic techniques are also an option. Regardless of surgical intervention, nodal evaluation is not recommended though still performed in as many as 25% of phyllodes cases (11). Hematogenous spread of these tumors makes axillary nodal sampling unnecessary even in the setting of malignant phyllodes unless there is clinical evidence of nodal involvement (11,12).
Prior recommendations mandating wide, 1 cm margins for BPTs have recently come under scrutiny. Multiple studies performed have investigated the potential for recurrence based on less than 1cm margins and have concluded the only prognostic indicator for recurrence is a positive margin and recurrence does not improve with wider margins (13-27) (Table 2). For this reason, a 1cm margin is no longer deemed the standard in effectively managing BPTs. In fact, approximately 87% of patients with BPTs and a focally positive margin will not experience recurrence (18). For patients with a BPT and a positive margin either re-excision or surveillance are acceptable options for management. The most recent National Comprehensive Care Network (NCCN) recommendations still recommend wide excision for borderline and malignant phyllodes tumors but note the inability to achieve a 1-cm margin with breast conservation is not an absolute indication for mastectomy (29). Adjuvant radiation has been shown to decrease local recurrence and may be indicated when a recurrence would cause significant morbidity (27,29,30).
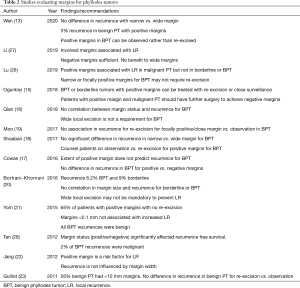
Full table
Recurrence
Historical data site recurrence as a relatively common phenomenon occurring in up to 21% of cases (12,31,32) while more contemporary data cite recurrence of 8–10% for benign, 13–14% for borderline and 18% for malignant phyllodes tumors (26,28).
An externally validated nomogram has been developed to predict likelihood of recurrence based on stromal atypia, mitosis, overgrowth and surgical margins (AMOS) (24,28,33,34). This provides a predictable manner to assess the likelihood for reoccurrence compared to tumor grading alone. This nomogram may be used to help guided decision making in initial treatment as well as the decision to re-excise versus for close surveillance in the setting of a positive margin.
Involved surgical margins is a well-documented independent prognostic factor in local recurrence (25,27,28). In the setting of distant metastasis, histologic factors such as high tumor grade, mitotic count, pleomorphism, overgrowth, infiltrative border and necrosis are more important than margin status (27). Though positive margins are a risk factor for local and distant recurrence, margin width has not been shown to impact recurrence rates (27,28). Additionally, genomic assays have demonstrated mutations present in borderline and malignant phyllodes tumors in proto oncogenes NF1, RB1, TP53, PIK3CA, ERBB4 and EGFR which may play a role in their higher rates of recurrence (35).
A meta-analysis assessing recurrence found 26% of BPTs and 21% of borderline tumors upgraded at recurrence noting the importance of close surveillance even in benign and borderline cases (26). It is important to note there was no pathology review as part of this meta-analysis and some cases were misclassified at initial presentation and falsely upgraded at recurrence. Time to recurrence varies with several studies but is generally within 3 years (17,26). After recurrence, efforts should be made for repeat excision to negative margins. Radiation is used with increasing frequency in borderline and malignant phyllodes with reports of improved local control (36).
Malignant phyllodes
Malignant phyllodes are extremely rare and no randomized trials exist in the treatment of phyllodes tumors. The NCCN recommendation for treatment of malignant phyllodes tumors is wide local excision with the intention of achieving 1 cm margins (29). Malignant phyllodes tumors behave histologically similar to sarcomas. Metastasis can be seen in up 22% of patients with malignant phyllodes at presentation with the most common site being lung followed by bone, heart and liver (37). Once a diagnosis of malignant phyllodes is made, staging computed topography (CT) of the chest is prudent to rule out pulmonary metastasis. Additional imaging may be warranted pending patient symptoms. Patients with metastasis have an overall poor prognosis, many dying within 3 years regardless of systemic therapy regimen (37). There is no survival benefit to surgical excision of the breast primary in the setting of metastatic disease; however, palliative surgery could be performed on an individualized basis for local control if feasible. Once metastasis has been excluded, surgical excision of the malignant tumor is recommended with wide 1 cm margins. Routine axillary nodal staging is not recommended for the effective treatment of phyllodes given low propensity for nodal spread (11,12,37).
Radiation is increasingly utilized for treatment of both borderline and malignant phyllodes tumors. Radiation has been effective in decreasing loco-regional recurrence with no change in overall survival (25,30). A meta-analysis has demonstrated improved local control in patients with breast conservation surgery and radiation regardless of margin status (38). Despite documented lower recurrence rates, radiation with breast conservation has not demonstrated improved cancer specific survival (CSS) (36). The data is scarce for mastectomy and radiation. Prior studies have demonstrated inferior outcomes in this patient population but this is likely due to selection bias with patients in these studies having more advanced tumors with poor prognostic features (36). Newer studies have demonstrated inferior, but not statistically significant, outcomes with mastectomy and radiation suggesting larger studies may further reduce the impact of selection bias (36). Overall, findings would suggest improved local control but overall no improvement in CSS with the use of adjuvant radiation (36-38).
The use of chemotherapy is uncertain given the high likelihood for recurrence and poor prognosis in metastatic disease. Doxorubicin with Dacarbazine compared to no medical therapy has been studied with no benefit in relapse free survival (39). For this reason, chemotherapy is not routinely recommended but may be considered on an individualized basis for large tumors or those with chest wall involvement. Chemo embolization has also been described in the literature for large, bulky tumors but lacks data for recommended routine use (40).
Conclusions
Phyllodes tumors are rare fibroepithelial lesions which can resemble benign fibroadenomas. The mainstay of treatment is complete surgical excision. BPTs with narrow or focally positive margins may be closely observed as opposed to routine margin re-excision (Table 2). Recurrence can be managed with wide local excision and radiation may be considered to improve local control for borderline and malignant subtypes. Data on systemic therapy for malignant phyllodes tumors is sparse and it is not routinely recommended.
Clinical scenario 1: fibroepithelial lesion
A 40-year-old female presents with an enlarged mass in the right breast. She has no prior imaging. A diagnostic mammogram and targeted ultrasound of the breast reveal a 4-cm lobulated breast mass. A core needle biopsy is performed which demonstrates a fibroepithelial lesion with increased stromal cellularity.
Question: What are the next steps in management?
Answer: This lesion have overlapping features of a cellular fibroadenoma and a phyllodes tumor. Surgical excision is the next step in management. Given the uncertainty of diagnosis, surgical excision should be performed similar to a fibroadenoma where the lesion is enucleated from the surrounding breast tissue.
The patient has the lesion surgically excised. The final pathology is consistent with a BPT measuring 4 cm in size. Multiple margins are positive on final pathology.
Question: What are the next steps in management?
Answer: The diagnosis of a phyllodes tumor was made after surgical excision. Positive margins are common after enucleation but may not reflect a true positive margin as the specimen surface may become damaged during surgery or in during pathology processing. This is supported in low recurrence rates and no residual tumor with re-excision in the setting of a “positive” margin (41). It may not be mandatory to re-excise an enucleated phyllodes tumor with benign morphology.
Clinical scenario 2: BPT
A 40-year-old female presents with a rapidly enlarged mass in the right breast. She has no prior breast imaging. She underwent diagnostic mammogram and targeted ultrasound of the right breast revealing a lobulated mass measuring 5 cm. A core needle biopsy was performed. Biopsy results demonstrate a fibroepithelial lesion, favor benign phyllodes.
Question: What are next steps for management?
Answer: Surgical excision is the mainstay of treatment. Depending on the size of the tumor relative to the volume of breast tissue, breast conservation with or without oncoplastic reduction would be the best management of this benign lesion. Surgical pathology reveals a BPT. The posterior margin is locally positive and all remaining margins are negative.
Question: What are the next steps in management?
Answer: A focally positive margin does not necessitate re-excision. Patient can be offered re-excision if feasible or close surveillance. If the patient develops a recurrence, excision is recommended.
Clinical scenario 3: malignant phyllodes tumor
A 40-year-old patient presents with a rapidly enlarging right breast mass. She has no prior imaging. A diagnostic mammogram and targeted ultrasound of the breast reveal a lobulated mass with internal clefts. A core needle biopsy was performed. Biopsy results demonstrate a fibroepithelial lesion suspicious for malignant phyllodes tumor.
Question: What are the next steps in management?
Answer: The patient has a malignant phyllodes tumor. A thorough history and physical to identify any symptoms that may suggest metastatic disease should be performed. A chest CT as well additional appropriate imaging guided by H&P should be performed to rule out metastatic disease. The H&P was unremarkable and CT of the chest is negative for metastatic disease.
Question: What are the next steps in management?
Answer: Wide local excision of the tumor is recommended. Breast conservation with oncoplastic reduction or mastectomy can be performed depending on tumor size and breast volume. Inability to achieve 1cm margins with breast conservation does not mandate a mastectomy. A sentinel lymph node biopsy is not recommended.
Wide local excision of the tumor with oncoplastic reduction is performed. The surgical margins are negative but less than 1 cm.
Question: What are the next steps in management?
Answer: Closure surveillance is recommended to assess for recurrence. Referral to radiation oncology is appropriate to discuss the benefits of adjuvant radiation therapy. There are no standard recommendations for adjuvant chemotherapy in the setting of malignant phyllodes tumors. This tumor has been excised to negative margins and is not invading the chest wall therefore there is no benefit to adjuvant chemotherapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Katharine Yao) for the series “A Practical Guide to Management of Benign Breast Disease” published Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-99). The series “A Practical Guide to Management of Benign Breast Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guerrero MA, Ballard BR, Brau AM, et al. Malignant phyllodes tumor of the breast: review of the literature and case report of stromal overgrowth. Surg Oncol 2003;12:27-37. [Crossref] [PubMed]
- Schnitt S, Collins L. Biopsy Interpretation of the Breast. Lippincott Williams and Wilkins, 2013:186-99.
- Tan PH, Tse G, Lee A, Simpson J, et al. Fibroepithelial tumours. In: Lakhani SR, Ellis IO, Schnitt SJ, et al. editors. WHO Classification of Tumours of the Breast. Lyon: IARC Press, 2012:142-7.
- Reinfuss M, Mitrus J, Duda K, et al. The treatment and prognosis of patient with phyllodes tumors of the breast: an analysis of 170 cases. Cancer 1996;77:910-6. [Crossref] [PubMed]
- Bernstein L, Deapen D, Ross Rk. The descriptive epidemiology of malignant cystosarcoma phyllodes tumors of the breast. Cancer 1993;71:3020-4. [Crossref] [PubMed]
- Lee AH, Hodi Z, Ellis IO, et al. Histological features useful in the distinction of phyllodes tumor and fibroadenomas on core needle biopsy of the breast. Histopathology 2007;51:336-44. [Crossref] [PubMed]
- Birch JM, Alston RD, McNally RJ, et al. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene 2001;20:4621-8. [Crossref] [PubMed]
- Wiratkapun C, Piyapan P, Lertsithichai P, et al. Fibroadenoma versus phyllodes tumor: distinguishing factors in patients diagnosed with fibroepithelial lesions after a core needle biopsy. Diagn Interv Radiol 2014;20:27-33. [Crossref] [PubMed]
- Tan BY, Geza A, Apple SK, et al. Phyllodes tumors of the breast: a consensus review. Histopathology 2016;68:5-21. [Crossref] [PubMed]
- Macdonald OK, Lee CM, Tward JD, et al. Malignant phyllodes tumor of the female breast: Association of primary therapy with cause-specific survival from the Surveillance, Epidemiology, and End Results (SEER) program. Cancer 2006;107:2127-33. [Crossref] [PubMed]
- Adesoye T, Beuman HB, Wilke LG, et al. Current Trends in the management of phyllodes tumors of the breast. Ann Surg Oncol 2016;23:3199-205. [Crossref] [PubMed]
- Mangi AA, Smith BL, Gadd MA, et al. Surgical management of phyllodes tumors. Arch Surg 1999;134:487-92; discussion 492-3. [Crossref] [PubMed]
- Wen B, Mousadoust D, Warburton R, et al. Phyllodes tumours of the breast: outcomes and recurrence after excision. Am J Surg 2020;219:790-4. [Crossref] [PubMed]
- Ditsatham C, Chongruksut W. Phyllodes tumor of the breast: diagnosis, management and outcome during a 10-year experience. Cancer Manag Res 2019;11:7805-11. [Crossref] [PubMed]
- Ogunbiyi S, Perry A, Jakate K, et al. Phyllodes tumor of the breast and margins: How much is enough? Can J Surg 2019;62:E19-21. [Crossref] [PubMed]
- Qian Y, Quan ML, Ogilvi T, et al. Surgical management of benign phyllodes tumours of the breast: Is wide local excision really necessary? Can J Surg 2018;61:17617. [Crossref] [PubMed]
- Cowan ML, Argani P, Cimino-Mathews A. Benign and low-grade fibroepithelial neoplasms of the breast have low recurrence reate after positive surgical margins. Mod Pathol 2016;29:259-65. [Crossref] [PubMed]
- Shaaban M, Barthelme L. Benign phyllodes tumour of the breast: (over) treatment of margins- a literature review. Eur J Surg Oncol 2017;43:1186-90. [Crossref] [PubMed]
- Moo TA, Alabdulkareem H, Tam A, et al. Association between recurrence and re-excision for close and positive margins versus observation in patients with benign phyllodes tumors. Ann Surg Oncol 2017;24:3088-92. [Crossref] [PubMed]
- Borhani-Khomani K, Talman M-LM, Kroman N, et al. Risk of local recurrence of benign and borderline phyllodes tumors: a Danish population=based retrospective study. Ann Surg Oncol 2016;23:1543-8. [Crossref] [PubMed]
- Yom CK, et al. Reappraisal of conventional risk stratification for local recurrence based on clinical outcomes in 284 resected phyllodes tumors of the breast. Ann Surg Oncol 2015;22:2912-8. [Crossref] [PubMed]
- Jang JH, et al. Clinicopathologic risk facors for the local recurrence of phyllodes tumors of the breast. Ann Surg Oncol 2012;19:2612-7. [Crossref] [PubMed]
- Guillot E, et al. Management of phyllodes breast tumors. Breast J 2011;17:129-37. [Crossref] [PubMed]
- Nishimura R, Tan PH, Thike AA, et al. Utility of the Singapore nomogram for predicting recurrence-free survival in Japanese women with breast phyllodes tumours. J Clin Pathol 2014;67:748-50. [Crossref] [PubMed]
- Gnerlich JL, Willimans RT, Kao K, et al. Utilization of radiotherapy for malignant phyllodes tumors: analysis of the National Cancer Database 1998-2009. Ann Surg Oncol 2014;21:1222-30. [Crossref] [PubMed]
- Lu Y, Yanbo C, Zhu L, et al. Local recurrence of benign, borderline, and malignant phyllodes tumors of the breast: a systematic review and meta-analysis. Ann Surg Oncol 2019;26:1263-75. [Crossref] [PubMed]
- Li J, Tsang JY, Chen C, et al. Predicting outcome in mammary phyllodes tumors: relevance of clinicopathological features. Ann Surg Oncol 2019;26:2747-58. [Crossref] [PubMed]
- Tan PH, Thike AA, Tan WJ, et al. Predicting clinical behavior of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol 2012;65:69-76. [Crossref] [PubMed]
- Nation Comprehensive Cancer Network. NCCN clinical breast guidelines in oncology. Breast cancer. Version 4.2020. 2020; Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed June 9, 2020.
- Barth RJJ, Wells WA, Mitchell SE, et al. A prospective, multi-institutional study of adjuvant radiotherapy after resection of malignant phyllodes tumors. Ann Surg Oncol 2009;16:2288-94. [Crossref] [PubMed]
- Barth RJJ. Histologic features predict local recurrence after breast conserving therapy of phyllodes tumors. Breast Cancer Res Treat 1999;57:291-5. [Crossref] [PubMed]
- Lakhani S, Ellis I, Schnitt S, et al. WHO classification of tumours of the breast. Geneva: World Health Organization, 2012.
- Chng TW, Gudi M, Lim SH, et al. Validate of the Singapore nomogram for outcome prediction in breast phyllodes tumors in a large patient cohort. J Clin Pathol 2018;71:125-8. [Crossref] [PubMed]
- Chng TW, Lee JY, Lee CS, et al. Validation of the Singapore nomogram for outcome prediction in breast phyllodes tumours: an Australian cohort. J Clin Pathol 2016;69:1124-6. [Crossref] [PubMed]
- Tan J, Ong CK, Lim WK, et al. Genomic landscapes of breast fibroepithelial tumors. Nat Genet 2015;47:1341-5. [Crossref] [PubMed]
- Kim YJ, Kim K. Radiation therapy for malignant phyllodes tumor of the breast: An analysis of SEER data. Breast 2017;32:26-32. [Crossref] [PubMed]
- Strode M, Khoury T, Mangieri C, et al. Update on the diagnosis and management of malignant phyllodes tumors of the breast. Breast 2017;33:91-6. [Crossref] [PubMed]
- Zeng S, Zhang X, Yang D, et al. Effects of adjuvant radiotherapy on borderline and malignant phyllodes tumors: a systematic review and meta-analysis. Mol Clin Oncol 2015;3:663-71. [Crossref] [PubMed]
- Morales-Vásquez F, Gonzelez-Angula AM, Broglio K, et al. Adjuvant chemotherapy with doxorubicin and dacarbazine has no effect in recurrence-free survival of malignant phyllodes tumors of the breast. Breast J 2007;13:551-6. [Crossref] [PubMed]
- Hashimoto K, Mimura H, Arai Y, et al. Successful preoperative chemoembolization in the treatment of a giant malignant phyllodes tumor. Cardiovasc Intervent Radiol 2016;39:1070-5. [Crossref] [PubMed]
- Slodkowska E, Nofech-Mozes S, Xu B, et al. Fibroepithelial lesions of the breast: a comprehensive morphological and outcome analysis of a large series. Mod Pathol 2018;31:1073-84. [Crossref] [PubMed]
Cite this article as: Simpson A, Li P, Dietz J. Diagnosis and management of phyllodes tumors of the breast. Ann Breast Surg 2021;5:8.

