
Langer axillary arch in breast surgery: a narrative review
Introduction
The axillary surgery plays a fundamental role in the treatment of breast cancer, melanoma of the trunk and the upper limb, in the histological diagnosis of lymphoma and in all those cases where it is necessary to remove for diagnostic purposes both lymph nodes (LNs), and axillary masses of unclear nature.
The axillary surgery for breast cancer has further stimulated the interest in Langer’s axillary arch (LAA) that was described as an extensive sheet of skin-associated musculature between the superficial fascia and the subcutaneous fat (1). Initially reported as muscle variation in the axillary fossa by Bugnone in 1783 (2), the “axillary arch” was identified by Ramsay in 1795 (3). However, it was Langer in 1846 who explained the axillary muscle more accurately, being aware of tension lines in the skin which were recognized as potentially related to surgical incisions (4,5). Variants of LAA with possible structure and position in relation to muscles, nerves, and axillary vessels have been further described (6-9). LAA is usually asymptomatic, but it could be implicated in the syndrome of costoclavicular compression and of hyper abduction, thoracic outlet and shoulder instability, as like as in the entrapment of the median nerve; further it can present with upper limb deep vein thrombosis or venous congestive symptoms (10,11).
Several data about morphological terminology and frequency of LAA can be obtained from the study by Jelev et al. (6) along with the clinical significance of axillary arch which presents some peculiar features to be taken into account during procedures of axillary surgery. Despite this, a recent meta-analysis (12) outlined that the majority of studies have been conducted on cadavers. Consequently, the possibility to calculate the total prevalence of the atypical muscles in the general population comes from cadaveric studies (13), more than from surgical axillary procedures (12). The occurrence of LAA is at 7–8%, varying frequencies from 0.25–43.8% depending on the population studied. Probably on account of most of the axillary surgery applied to women, the reported occurrence of the LAA is major on female than male patients, bilateral or unilateral as well (3,6,8,14).
Although intraoperative recognition of muscle variations in a given anatomical region does not necessarily reflect its surgical significance (9), the failure to report or to identify the accessory muscles of the axilla undoubtedly affects their clinical significance so that even experienced breast or thoracic surgeons are expected to deliver a detailed knowledge of the axilla (8,9,15-18).
Therefore, this review seeks to explore the literature on LAA in breast surgery along with features of the Decision Support System (DSS) used by surgeons (15) to identify whether the LAA might be preserved or resected. We present the following article in accordance with the Narrative review reporting checklist (available at http://dx.doi.org/10.21037/abs-20-115).
Methods
Search strategy
The major electronic database (PubMed, Web of Science, and Scopus) were thoroughly searched for studies on ‘breast surgery’, ‘Langer’s axillary arch’, ‘lymphadenectomy’, ‘dissection’, ‘sentinel lymph node’, and ‘preoperatively’. Following acquisition of the full texts, other potentially eligible articles that could have been missing in the electronic databases’ search were screened with a reference search. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (19) was applied in scrutinising references of relevant articles for pertinent studies. Articles which did not identify the LAA in breast surgery were excluded. The search was carried out in August 2020 with no prior time limit set for inclusion of data as well as inclusion of every original language of publication.
Study endpoints
The primary endpoint was finding the capability of pre-operatively and intra-operatively techniques to detect the LAA in breast surgery. The secondary endpoint included surgeon DSS while encountering LAA during axillary access and examining features associated with the arch.
Results
Literature search
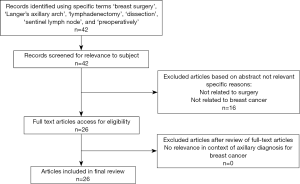
There was a total of 42 potential citations from the initial database search (Figure 1). Amongst these, articles discussed only axillary dissection in cadavers and anatomical studies on LAA along with studies examining LAA in other surgery than the ones for breast cancer were excluded. In total, 26 articles discussing relevant issues pertaining to LAA in breast surgery were included in this review. LAA occurrence during breast surgery either in sentinel lymph node biopsy (SLNB) or in axillary lymph node dissection (ALND) along with LAA treatment is described in 17 studies (Table 1). Other four papers evaluated the preoperative investigation of the axillary arch, whilst the remaining five articles described implications of LAA in breast surgical procedures like SLNB and ALND. Due to the few studies concerning breast surgery and management of LAA in clinical setting, a narrative review was performed.
Full table
Discussion
Preoperatively diagnosis of LAA
Preoperative diagnosis of the axillary arch supplies pieces of information for intraoperative navigation of SLNB, even if it appears to be difficult as this muscular variation crosses in front of the great axillary vessels and the first intercostal brachial nerve. Since LAA may hide the LNs of the first level of Berg (Figure 2), it can be confused with enlarged LNs or soft tissues tumours (8,9,20,21). Intermittent compression of the axillary vein or hyperabduction syndrome could be indicative for LAA presence (10,11,18).
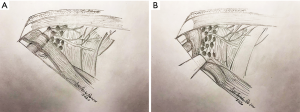
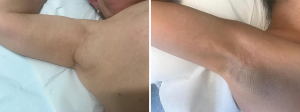
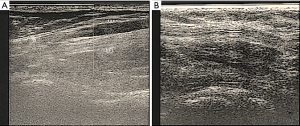
Sometimes, the arch could be easily visible at clinicjudgment (Figure 3). Imaging analysis, as like as mammography, echography, computed tomography (CT) and magnetic resonance imaging (MRI) have demonstrated to detect the presence of the axillary arch only in few clinical reports (22,31-35). Ko et al. (32) detailed on the capability of mammography to get imagines about a band-like structure overlapped with pectoralis muscle which they revealed to be the axillary arch. Keshtgar et al. reported that the LAA is fully stretched during imaging, so that the echography scanning was misleading in showing the location of the SLN (22). Indeed, echography examination could be more easily provided after surgery as an attempt to assess if a mass due to LAA could be resolved in patients where LAA is preserved (Figure 4). Anyway, at ultrasonography inspection, the muscular arch seemed to appear as a grey oval structure (15).
Multidetector row CT (MDCT) and MRI may allow at obtaining LAA recognition preoperatively (14,21,31). Rajakulasingam & Saifuddin reported MRI images with two slips of muscle arising from the anterior margin of the left latissimus dorsi muscle (33). The thicker slip crosses antero-inferior to the axillary neurovascular bundle without any compression or displacement. Guy et al. characterized at first the prevalence, anatomic relations, possible LN concealment, and potential neurovascular impingement of the axillary arch muscle in an extensive review of shoulder MRI data (34). They reported that the LAA was detected or excluded best on oblique coronal images more with fast spin-echo T2-weighted images than on spin-echo T1-weighted images thanks to the signal intensity contrast between LAA and fat. For a preoperative diagnosis with MRI, Suzuma et al. described the anatomical site of LAA takes origin at the anterior edge of the latissimus dorsi in the middle of the posterior fold of the axilla and tapering to a narrow tendon which was inserted into the posterior aspect of the trilaminar tendon of the pectoralis major (14).
Hong et al. presented the submission to MDCT of a case with a palpable, non-tender mass in left axilla with complains of intermittent ulnar numbness in left arm to forearm, aggravated with hyperabduction and relieved with adduction of the arm. The MDCT was able to discriminate between right and left axilla where a nodular elongated soft tissue structure was detected (35). With the same imaging technique, Ando et al. reported not significant different numbers of MDCT-LNs and failure rates of SLN identification between patients with or without the LAA (21).
LAA in SLNB
The presence of LAA might affect the SLNB. Keshtgar et al. reported that the SLN localization was extremely easy when the node was lying over the arch, whilst if the SLN was underneath LAA the node detection was more difficult (22). Ando et al. detailed the anatomical localization of LNs with respect to the common position of a SLN in a group of 56 patients with a diagnosis of axillary arch and SLNs identification (21). In some cases, SLNs were located in more lateral and superficial axillary positions, in others they were allocated in more cranial positions or in more dorsal positions or below anomalous muscles of the axilla. Examining eight patients over 3 years, Abudhaise et al. found sentinel LNs laterally to the LAA in the sub-pectoral region and associated with stretching of the efferent lymphatic vessels (23).
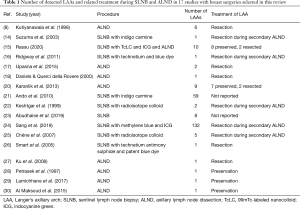
Few pieces of information are currently available in literature (Table 1) on the most useful tracers to be used during axillary access to avoid mistakes due to LAA. Inadequate clearance of Level 1 nodes due to them being partially covered by the arch (Figure 2A) could lead relatively inexperienced surgeons to a level above the axillary vein, with an increased risk of lymphedema of the arm postoperatively. Besides, as the LAA could act as a potential site of metastatic LNs (24) accurate identification by means of intraoperative localization with standardised techniques and performing tracers is strongly advised (15,16,24).
Ridgway et al. reported that percutaneous radioisotope counts are commonly maximal at the inferior edge of the axillary hairline just posterior to the pectoralis muscle in usual SLNB (16). In their experience, the maximal counts were more cranial and lateral than the common location of the sentinel nodes when LAA occurred. Further, they found no difficulty in finding LNs under an arch when radioisotope was used, even if an arch could conceal the nodes when blue dye was applied. In his paper investigating SLNB with 99mTc-labeled nanocolloid (TcLC) and indocyanine green (ICG) as tracers, Rassu (15) outlined that in the patient group with only SLNB, a mean number of 2.42 (±1.76) and 2.42 (±1.83) LNs per patient were harvested with TcLC and ICG, respectively. Similarly, the metastatic LNs were not significantly different: 0.08 (±0.34) with TcLC and 0.14 (±0.55) with ICG (15) so to corroborate the ability of radioisotope colloids “technetium-based” to provide accuracy in collecting LNs even in presence of anomalous muscular structure in the axilla (16,22,25).
The ICG tracer was evaluated along with methylene blue only in another study to identify LNs when masked by LAA (24). Literature reports evidence that SLNB using the fluorescent dye ICG allows at detecting LNs non-invasively with high accuracy and sensitivity (36). Indeed, Rassu outlined the meaningful advantage recorded applying the ICG tracer to map lymphatic vessels in order to minimize the confounding factor given by the anatomical limits of the operative field in case of Langer’s anatomical variation (15).
LAA in lymphadenectomy
The surgeon awareness of the anatomical variations that may appear in a specific axillary region is the leading factor for their intraoperative recognition along with their main anatomical features (9). During lymphadenectomy in LAA’s patients, Rassu outlined the axillary location that contains the vast majority of the LNs as almost fully covered by the arch (15): thus, amplifies the recurrence risk from undissected axillopectoral muscles harbouring positive nodes. Indeed, a very recent work confirmed that LAA’s LN has a relatively high metastasis rate (24), but the same research did not provide any comparison between LN status of patients with and without LAA as it was provided previously by Smart et al. (26) and more recently by Rassu (15) who observed no metastatic LNs in LAA patients even in secondary ALND.
In their systematic review of medical literature published between 1996 and 1999, Babu & Khashaba concluded that LAA should be recognized in axillary dissection to avoid confusion in staging of LNs and to prevent injury to axillary vessels and brachial plexus (37). The LAA might restrict axillary access during ALND as it is tautened by abduction and elevation of the arm: thus, allowing free mobility and relaxation of the muscle is therefore advocated (26).
Conclusions
LAA and features of the decision supporting system in breast surgery
The presence of the LAA muscular structure on the lateral side of the surgical field may be mistakenly confused with the latissimus dorsi muscle (Figure 2A). In this situation, the band that adheres to the medial edge of the muscle could not be recognized and the dissection might be continued along in a wrong plan too cranial than the axillary vein. As a consequence, the cords of the brachial plexus would be exposed to the risk to be injured with impairment of breast reconstruction (1,8,14,18,20,26) even after a radical mastectomy (27).
On the other hand, the LAA may complicate procedures as the SLNB and the lymphadenectomy giving risks as like as suboptimal staging and limited regional control of disease (1,16,17,20-22,24,26).
Accordingly, in order to get an optimal axillary clearance, research commonly suggests the cutting of this anomalous axillary muscle (Table 1). In detail, authors who support such a decision consider performing the LAA resection because of the recurrence risk to undissected axillopectoral muscles harbouring positive nodes is larger in magnitude (14,24). With a procedure of LAA division, as illustrated in Figure 2B, Ku et al. obtained a complete dissection of the medial and lateral axillary group with no evidence of axillary recurrence, lymphedema or any limitation of motion of the right arm after 2-year follow-up (27).
Another reason for muscle resection is based on the association of LAA with neurovascular compression leading to postoperative upper limb lymphedema and thoracic outlet syndrome with compression particularly noted during abduction or external rotation of the arm (18,20,25).
On the other hand, especially whether the LAA does not reveal to be problematic for the patient in order to permit later reconstructive surgery (1,8,14,18,20,26) the preservation of the neurovascular elements should continuously be taken into consideration by the surgeon (15). Gentle dissection of tissues with a reluctance to divide any horizontally lying veins should allow this anomaly to be identified and prevent inadvertent damage (8,38). This suggested to preserve the arch in certain LAA patients in line with the “SLNB era” advocated by Veronesi et al. (39). In two studies (15,20), the LAA was kept (Table 1). Indeed, in a mean 23 months follow-up carried out by Petrasek et al. there were no known complications related to the axillary arch, such as lymphedema, brachial plexus injury, axillary vein injury or thrombosis (28). The same uneventful procedure was provided by Lamichhane et al. (29) and Al Maksoud et al. (30) who were able to dissect nodes beneath the arch without the need to divide it.
Thus, breast surgeon’s awareness of the possible complications of an axillary arch muscle, the understanding of any functional deficit from it, along with their clinical judgment targeted for each patient may continue to stimulate the debate on the preservation or the cutting of axillary arches. Karanlik et al. and Rassu asserted their clinical judgment in a DSS to determine how to identify LAA, to question whether the axillary arch could be perceived as potentially problematic for the patient, and to handle results to the final decision (15,20). Such DSS approach, based on multiple variables, could motivate more research on clinical features of LAA in breast surgery where also patient age and status should be taken into account.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative review reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-115
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-115). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Besana-Ciani I, Greenall MJ. Langer's axillary arch: anatomy, embryological features and surgical implications. Surgeon 2005;3:325-7. [Crossref] [PubMed]
- Pitzorno H. Contributo alla morfologia dell’arco ascellare muscolare di Langer. Arch Ital Anat Embryol 1911;10:129-44.
- Ramsay A. An account of unusual conformation of some muscles and vessels. Edinb Med Surg J 1812;8:281-3. [PubMed]
- Langer C. Zur Anatomie Des Musculus Latissimus Dorsi. Osterreichische Med Wocheschrift 1846;15:454-8.
- Carmichael SW. The tangled web of Langer’s lines. Clin Anat 2014;27:162-8. [Crossref] [PubMed]
- Jelev L, Georgiev GP, Surchev L. Axillary arch in human: common morphology and variety. Definition of “clinical” axillary arch and its classification. Ann Anat 2007;189:473-81. [Crossref] [PubMed]
- Bertone VH, Ottone NE, Lo Tartaro M, et al. The morphology and clinical importance of the axillary arch. Folia Morphol (Warsz) 2008;67:261-6. [PubMed]
- Kutiyanawala MA, Stotter A, Windle R. Anatomical variants during axillary dissection. Br J Surg 1998;85:393-4. [Crossref] [PubMed]
- Natsis K, Vlasis K, Totlis T, et al. Abnormal muscles that may affect axillary lymphadenectomy: surgical anatomy. Breast Cancer Res Treat 2010;120:77-82. [Crossref] [PubMed]
- Magee C, Claire Jones MB, McIntosh S, et al. Upper limb deep vein thrombosis due to Langer’s axillary arch. J Vasc Surg 2012;55:234-6. [Crossref] [PubMed]
- Clarys JP, Barbaix E, Van Rompaey H, et al. The muscular arch of the axilla revisited: its possible role in the thoracic outlet and shoulder instability syndromes. Man Ther 1996;1:133-9. [Crossref] [PubMed]
- Taterra D, Henry BM, Zarzecki MP, et al. Prevalence and anatomy of the axillary arch and its implications in surgical practice: a meta-analysis. Surgeon 2019;17:43-51. [Crossref] [PubMed]
- Douvetzemis S, Natsis K, Piagkou M, et al. Accessory muscles of the anterior thoracic wall and axilla. Cadaveric, surgical and radiological incidence and clinical significance during breast and axillary surgery. Folia Morphol (Warsz) 2019;78:606-16. [Crossref] [PubMed]
- Suzuma T, Sakurai T, Yoshimura G, et al. Magnetic resonance axillography for preoperative diagnosis of the axillopectoral muscle (Langer’s axillary arch): a case report. Breast Cancer 2003;10:281-3. [Crossref] [PubMed]
- Rassu PC. A single-center study on 12 year-experience in lymphadenectomy and in sentinel lymph-node biopsy with 99mTc-labeled nanocolloid and indocyanine green as tracers: relationships with detection and management of the Langer’s axillary arch. Breast J 2020;26:1056-60. [Crossref] [PubMed]
- Ridgway PF, Collins AM, McCready DR. The surgical importance of an axillary arch in sentinel node biopsy. Surg Radiol Anat 2011;33:147-9. [Crossref] [PubMed]
- Upasna Kumar A. Muscular variations during axillary dissection: A clinical study in fifty patients. Niger J Surg 2015;21:60-2. [Crossref] [PubMed]
- Daniels IR, Querci della Rovere G. The axillary arch of Langer--the most common muscular variation in the axilla. Breast Cancer Res Treat 2000;59:77-80. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Karanlik H, Fathalizadeh A, Ilhan B, et al. Axillary arch may affect axillary lymphadenectomy. Breast Care (Basel) 2013;8:424-7. [Crossref] [PubMed]
- Ando J, Kitamura T, Kuroki Y, et al. Preoperative diagnosis of the axillary arch with multidetector row computed tomography and the axillary arch in association with anatomical problems of sentinel lymph node biopsy. Breast Cancer 2010;17:3-8. [Crossref] [PubMed]
- Keshtgar MRS, Saunders C, Ell PJ, et al. Langer’s axillary arch in association with sentinel lymph node. Breast 1999;8:152-3. [Crossref] [PubMed]
- Abudhaise H, Merh R, Devalia H. The anatomical relationship between the xillary arch of Langer and sentinel lymph node in breast cancer surgery. Ann R Coll Surg Engl 2019;101:533. [Crossref] [PubMed]
- Sang Y, Kong X, Li X, et al. Langer’s axillary arch lymph node metastatis in breast cancer patients: A prospective clinical study. Surg Oncol 2019;29:48-52. [Crossref] [PubMed]
- Chêne G, Le Bouëdec G, Dauplat G. Arch and sentinel: surgical technique of sentinel node biopsy with the axillopectoral muscle. Gynecol Obstet Fertil 2007;35:25-9. [PubMed]
- Smart PJ, Shayan R, Mann GB. Axillopectoral muscle: an important anomaly in axillary surgery. Surgical Practice 2005;9:147-9. [Crossref]
- Ku SK, Sang AH, Sairhee K, et al. The axillary arch of Langer (axillopectoral muscle): a case report. J Breast Cancer 2008;11:106-8. [Crossref]
- Petrasek AJ, Semple JL, McCready DR. The surgical and oncologic significance of the axillary arch during axillary lymphadenectomy. Can J Surg 1997;40:44-7. [PubMed]
- Lamichhane D, Agrawal SK, Mukhopadhyay S, et al. Axillary arch: clinical significance in breast cancer patients. Int J Case Rep Images 2017;8:758-61. [Crossref]
- Al Maksoud AM, Barsoum AK, Moneer MM. Langer’s arch: a rare anomaly affects axillary lymphadenectomy. J Surg Case Rep 2015;2015:rjv159 [Crossref] [PubMed]
- Koberlein GC, Hoffmann C. Langer's axillary arch: a frequent but rarely discussed anatomical variant in the radiologic literature. Pediatr Radiol 2018;48:433-6. [Crossref] [PubMed]
- Ko K, Han BK, Shin JH, et al. The axillopectoral muscle seen on mammography. Clin Radiol. 2006;61:625-9. [Crossref] [PubMed]
- Rajakulasingam R, Saifuddin A. Fullness in the left axilla-answer: Langer's axillary arch. Skeletal Radiol 2020;49:1677-9. [Crossref] [PubMed]
- Guy MS, Sandhu SK, Gowdy JM, et al. MRI of the axillary arch muscle: prevalence, anatomic relations, and potential consequences. AJR Am J Roentgenol 2011;196:W52-7. [Crossref] [PubMed]
- Hong HJ, Choi NJ, Han DH, et al. Axillary arch: detailed ultrasonographic images with multiplanar CT correlation. J Med Ultrason (2001) 2015;42:121-5. [PubMed]
- Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol 2009;16:2943-52. [Crossref] [PubMed]
- Babu ED, Khashaba A. Axillary arch and its implications in axillary dissection--a review. Int J Clin Pract 2000;54:524-5. [PubMed]
- Ivanovic N, Granic M, Randjelovic T, et al. Fragmentation of axillary fibrofatty tissue during dissection facilitates preservation of the intercostobrachial nerve and the lateral thoracic vein. Breast 2008;17:293-5. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546-53. [Crossref] [PubMed]
Cite this article as: Rassu PC. Langer axillary arch in breast surgery: a narrative review. Ann Breast Surg 2021;5:7.

