
The surgeon’s guide to fibroadenomas
Introduction
Fibroadenomas (FA) are the most common benign breast lesion. The true incidence of FA is difficult to assess since many of these patients are followed by imaging or clinical exam in their primary care physician’s office. However, autopsy studies show approximately 20 percent of women in adolescence to mid-20’s have FA (1). FA account for approximately half of all breast biopsies and are most commonly diagnosed in women between 15 and 35 years old (2). The risk of FA decreases significantly with age after the peak incidence in the 20–30 age group (3). Many FA patients are referred to breast surgery clinic. Therefore, as a modern breast surgeon, it is critically important to understand the appropriate work-up and management for this type of patient.
Definitions and breast cancer risk
FAs usually grow as sharply circumscribed spherical nodules and they are made up of epithelial and stromal components (4,5). FA are characterized as proliferative breast lesions without atypia and they are associated with a slight increased risk of developing breast cancer in the future, however there is some variation in risk based on subtype (6). The common subtypes of FA are juvenile, simple, complex, and giant.
Juvenile FA occur in young women between ages 10 and 18 and compromise 8% of all FAs; they present with an accelerated growth pattern (7). At the time of diagnosis, up to 25% of juvenile FA patients will have multiple or bilateral tumors (8). Simple FA represent approximately 86% of all FA’s and most often present as a palpable mass (9). The majority of women with simple FA and no family history of breast cancer are not at an increased risk of breast cancer in the future (10,11). Complex FA are FA with associated cysts, sclerosing adenosis, epithelial calcifications, or papillary apocrine changes and they represent approximately 14% of FA (9,11). Breast cancer risk in patients with complex FA is increased if there are proliferative changes in the surrounding breast tissue and complex FA alone is not considered an independent risk factor (9). Giant FA are greater than 5 cm in size and comprise 0.5–2% of all FA (12). There is rapid growth noted in giant FA and tissue diagnosis is necessary to rule out the possibility of a phyllodes tumor (13). Due to the size of these lesions and the diagnostic dilemma differentiating giant FA from a phyllodes tumor, surgical excision is the mainstay of treatment (14).
Risk factors for FA
The exact etiology of FA is unknown. There is likely a hormonal component because FA are most common during the reproductive years and they also often enlarge during pregnancy or with estrogen therapies. Risk factors that increase a women’s risk for breast cancer also seem to increase her risk for FA, including early menarche in some reports (15). A family history of breast cancer has also been shown to increase the risk of FA in certain women (16). A case-control study from China showed a significant decreased risk of FA with increased intake of fruits and vegetables as well as oral contraceptive use (17). However, other studies have not demonstrated a linkage between FA and age at menarche, age at menopause, or hormonal therapy, including oral contraceptives (18).
Workup
Breast complaints compromise at least 3% of women’s visits with their general practice physician and an increasing number of these patients are referred to a specialized breast clinic (19,20). As a breast surgeon, the work-up of a new breast mass can be classified as clinical, imaging assessment, and pathologic examination (21). Using all three of these processes in a complementary and coordinated fashion ensures that patients get the highest level of care without unnecessary interventions.
Clinical
FA usually have the presenting symptom of a breast mass. Key history points that help differentiate this within the broad differential of breast mass include duration of symptoms and severity. If there is any fluctuation in size of the mass with the menstrual cycle, this mass is more likely to be a cyst (22). A history of trauma or surgery to the breast is often associated with fat necrosis (23). Associated symptoms such as skin changes or nipple discharge decrease the likelihood the mass is a FA. A focused history of prior breast biopsies and surgeries as well as family history of cancer will aid in assessing risk factors for FA.
On clinical exam, key areas of focus are the lymph node basins (specifically cervical, supraclavicular, infraclavicular, and axillary) and breast exam. Careful visual exam of the patient’s breasts with the arms at the sides and raised above the head help highlight cosmetic deformity caused by an underlying breast mass. The lymph node exam should be performed with the patient seated. The breast exam should be performed in both the seated and supine positions for greatest accuracy (24). FA usually present as a well-defined mobile mass in the breast without overlying skin changes or nipple discharge. Lymph node examination would be expected to be normal for these patients.
Imaging assessment
Imaging assessment of a new breast mass is necessary in essentially all patients because the mass may not exhibit distinctive physical findings. It is preferable for imaging to occur prior to biopsy as the biopsy changes may obscure the imaging interpretation. The American College of Radiology Appropriateness Criteria® for palpable breast masses outlines that for a clinically detected palpable breast mass, the patient’s age dictates the recommended first imaging modality. If a patient is 40 or older, she should start with diagnostic mammography. If she is younger than 30, the first imaging option is a breast ultrasound (US). If the patient is between 30 and 39, either US or diagnostic mammography are reasonable as initial imaging. Any highly suspicious breast mass should be biopsied, regardless of imaging findings (25).
US should be performed using a high-resolution, real-time, linear array scanner with a minimum frequency of 10 MHz (25). The US should be directed to the palpable mass (26). On US, FA are most commonly described as a hypoechoic mass with a circumscribed border (27). Diagnostic mammogram usually consists of a craniocaudal (CC) and mediolateral oblique (MLO) view of each breast. A small radiopaque marker is placed on the skin overlying the mass to aid in the ability to obtain spot compression or magnification views (25). Mammogram demonstrates an oval or round mass with a circumscribed margin (28). Calcifications are occasionally seen associated with an involuting FA, often in post-menopausal women, and are usually coarse and “popcorn-like” (7). Breast magnetic resonance imaging (MRI) is occasionally used in the work-up of FA, especially if there are multiple breast masses and biopsy of all the findings would be difficult (29). The appearance of FA on MRI varies based on the hyalinization of the lesion. T2 hypointensity is seen with sclerotic or hyalinized FA while T2 hyperintensity is seen with cellular FA. FA also show varied enhancement patterns however typical FA show rapid initial and persistent delayed phases, also called type 1 enhancement kinetics (30).
Pathologic examination
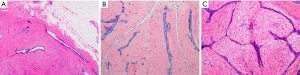
Clinical concern and imaging characteristics determine the need for pathologic examination in suspected FA. Younger patients, under age 40, with a Breast Imaging-Reporting and Data System (BIRADS) 3 lesion can often be safely followed with careful surveillance. Women over age 40 with palpable breast masses, even with benign features on imaging, and all women with a BIRADS 4 or higher finding should be considered for biopsy (31). The ideal approach is a percutaneous core biopsy. FA classically have an evenly distributed glandular and stromal elements ratio and the borders of the lesion are usually circumscribed and pushing, without infiltrating the surrounding tissue. The stroma is typically low in cellularity and does not have significant nuclear atypia. The epithelial component shows an intact myoepithelial layer supporting ductal epithelial cells (32) (Figure 1). Percutaneous core needle biopsy is recommended over a fine needle aspiration as it is more accurate in differentiating between a FA and a phyllodes tumor (33).
Management options
Once the breast surgeon has diagnosed an FA in their patient, the next step is discussing a management strategy; surveillance, surgical excision, or alternative management (Table 1).
Table 1
| Author | Type of study | Number of subjects | Average patient age | Key point(s) |
|---|---|---|---|---|
| Surveillance | ||||
| McLaughlin (34) | Retrospective chart review | 196 | 15 | Observation is appropriate for asymptomatic breast masses in children |
| Rao (35) | Evidence based guidelines | N/A | N/A | No routine excision of <2 cm FA |
| Harvey (36) | Evidence based guidelines | N/A | N/A | Short term imaging follow up for benign appearing masses |
| Surgical excision | ||||
| Dialani (37) | Retrospective review | 378 | Not reported | Even FA that are enlarging on imaging are highly unlikely to be malignant |
| Hubbard (38) | Retrospective review | 723 | 32 | Surgery for FA if patient >35 years old, FA >2.5 cm, or poorly circumscribed mass |
| Alternative management | ||||
| Kaufman (39) | Prospective nonrandomized trial | 47 | 34 | At 12 months of follow-up following cryoablation, 75% of FA were non-palpable |
| Li (40) | Retrospective review | 1,578 | 35 | 3% recurrence risk, associated with larger lesion size, using vacuum-assisted percutaneous excision |
| Kovatcheva (41) | Prospective nonrandomized trial | 42 | 32 | US guided HIFU shows a 72.5% mean volume reduction of the FA at 12-month follow-up |
FA, fibroadenoma; US, ultrasound; HIFU, high-intensity focused ultrasound.
Surveillance
Observation alone is reasonable in pediatric FA that are asymptomatic (34). In adult patients, the American Society of Breast Surgeons Choosing Wisely® campaign recommends against routinely excising biopsy-proven FA that are <2 cm (35). The American College of Radiology Appropriateness Criteria® for palpable breast masses even states that short term imaging follow-up (such as every 6 months for 2 years) is a reasonable alternative to biopsy for solid masses with probably benign features suggesting FA (36).
Surgical excision
Defining which patients require surgery for biopsy proven FA can be difficult and it requires the breast surgeon to take an individualized case-based approach. Some earlier data drove a more aggressive stance, recommending surgical excision for patients >35 years old, immobile or poorly circumscribed masses, and FA size greater than 2.5 cm (38). If a biopsy proven FA is enlarging on imaging or clinical follow-up, there is still a very low risk of malignancy (42). For biopsy proven FA, surgical excision is should be considered if there is associated atypia, unusual pathologic features, or symptomatic/cosmetic concerns (37).
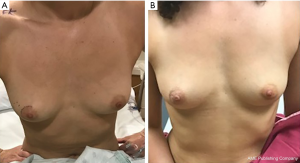
The surgical removal of a biopsy proven FA is considered an excisional biopsy, which the American Medical Association assigns Current Procedural Terminology (CPT) code 19120 for billing purposes. This surgery can often be done using sedation and local anesthesia however occasionally requires general anesthesia, especially with larger lesions. Perioperative antibiotics are not required for these cases (43). For a palpable lesion without a radiologic marker, it is important for the patient and the surgeon to agree on the mass being removed and circle it on the skin pre-operatively, to avoid any confusion (Figure 2A). Aesthetic scar placement, one of the basic building blocks of oncoplastic breast surgery, is recommended to maximize the cosmetic outcomes (44). A biopsy proven FA can be safely enucleated and margins are not recommended (45). Even very large FA can often be enucleated and leave the patient with an excellent aesthetic result as long as surrounding breast parenchyma is not resected with the FA (Figure 2B). However, if there are concerns for long-term cosmetic outcomes, reconstructive plastic surgery should be consulted and a combination procedure can be considered (46).
Alternative management
Alternatives to surgical excision exist but they should only be considered in patients with a core biopsy proven FA. US guided cryoablation is one alternative for FA that has shown significant decrease in lesion size after treatment, with 75% of lesions being non-palpable at 1 year of follow-up (39). US guided vacuum-assisted percutaneous excision can also be performed for FA, with good patient satisfaction. Recurrence occurs approximately 4% of the time with this modality and is more likely in patients with multiple lesions, a larger lesion size, and a hematoma at the time of the procedure (40). There are preliminary studies using percutaneous US ablation for FA, such as high-intensity focused ultrasound (HIFU), that have shown promising results. One European multicenter study using HIFU showed a 72.5% volume reduction of the lesion at 1 year follow-up of the procedure, with minimal transient side effects (41). Clinical follow-up by the performing physician is recommended in these patients due to the lack of long-term data on these emerging technologies.
Recommendations
FA is an extremely common benign breast lesion affecting younger women. The ideal approach for a patient with a breast mass suggestive of FA is to image with US +/− mammogram, confirm the lesion is an FA using percutaneous core biopsy, and conservative follow-up in the future. If the FA is causing concern for the patient or the physician has suspicion for malignancy, surgical excision is reasonable and should be performed in an oncoplastic fashion to maximize long-term aesthetic outcomes (Table 2).
Table 2
| Initial imaging: ultrasound (mammogram if over 40 years old) |
| Biopsy modality: core biopsy |
| Asymptomatic FA management: observation |
| Symptomatic FA management: surgical excision |
| FA management with concern for malignancy: surgical excision |
FA, fibroadenoma.
Common clinical scenarios
- A 14-year-old female is referred to you by her pediatrician who found a small, mobile, 1.5 cm mass in the patient’s left breast on clinical exam. This is not bothering the patient. How would you manage this? Careful physical exam followed by ultrasound. If US shows imaging characteristics classic for FA, serial US every 6 months for 2 years and observation. If US shows concerning characteristics, perform core biopsy. If the pathology is FA, clinical surveillance is reasonable (47).
- A 26-year-old patient comes to your clinic with a rapidly enlarging 3.5 cm mass in her right breast that is visible in her swimsuit and causing her distress. What do you recommend? Physical exam to confirm this is the only breast abnormality and then US. Due to the size and rapid enlargement of the mass, core biopsy is necessary to rule out malignancy. Surgical excision should be considered due to the symptomatic nature and rapid growth pattern of this lesion even if only FA is diagnosed on core biopsy, as the imaging and pathologic distinctions of phyllodes tumor and FA are nuanced (32).
- A 45-year-old female presents with a screening mammogram finding of a 2.7 cm mass, the radiologist reports it has characteristics indicating it is likely a FA. She has no symptoms and a normal physical exam. What are your next steps? Diagnostic mammogram and US followed by an US guided core biopsy. The core biopsy shows a “fibroadenoma with associated atypia and radial scar”. Surgical excision, with imaging localization prior to surgery, is recommended due to the patient’s age, the lesion size, and the associated atypia (37,38).
Conclusions
Breast surgeons will see numerous FA patients in their clinic during their career. An appropriate work-up and discussion with the patient will help determine the best options for treatment or surveillance. The ideal management of this condition relies on using evidence-based guidelines and taking into account the patient’s preferences.
Acknowledgments
The authors would like to thank Dr. Megan Sullivan for her assistance with providing pathologic photographs for the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Breast Surgery for the series “A Practical Guide to Management of Benign Breast Disease”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-100). The series “A Practical Guide to Management of Benign Breast Disease” was commissioned by the editorial office without any funding or sponsorship. KY served as the unpaid Guest Editor of this series and serves as an unpaid editorial board member of Annals of Breast Surgery from Oct 2019 to Sept 2021. KK has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005;353:275-85. [Crossref] [PubMed]
- Carty NJ, Carter C, Rubin C, et al. Management of fibroadenoma of the breast. Ann R Coll Surg Engl 1995;77:127-30. [PubMed]
- Li J, Humphreys K, Ho PJ, et al. Family History, Reproductive, and Lifestyle Risk Factors for Fibroadenoma and Breast Cancer. JNCI Cancer Spectr 2018;2:pky051 [Crossref] [PubMed]
- Kumar V, Abbas AK, Aster JC. Robbins and Cotran pathologic basis of disease. Ninth edition. ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Krings G, Bean GR, Chen YY. Fibroepithelial lesions; The WHO spectrum. Semin Diagn Pathol 2017;34:438-52. [Crossref] [PubMed]
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005;353:229-37. [Crossref] [PubMed]
- Goel NB, Knight TE, Pandey S, et al. Fibrous lesions of the breast: imaging-pathologic correlation. Radiographics 2005;25:1547-59. [Crossref] [PubMed]
- Roveda D Junior, Badan GM, Campos M, et al. Juvenile fibroadenoma. Radiol Bras 2018;51:136-7. [Crossref] [PubMed]
- Nassar A, Visscher DW, Degnim AC, et al. Complex fibroadenoma and breast cancer risk: a Mayo Clinic Benign Breast Disease Cohort Study. Breast Cancer Res Treat 2015;153:397-405. [Crossref] [PubMed]
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985;312:146-51. [Crossref] [PubMed]
- Dupont WD, Page DL, Parl FF, et al. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med 1994;331:10-5. [Crossref] [PubMed]
- Dolmans GH, Hoogbergen MM, van Rappard JH. Giant fibroadenoma of one breast: Immediate bilateral reconstruction. J Plast Reconstr Aesthet Surg 2007;60:1156-7. [Crossref] [PubMed]
- Yagnik VD. Juvenile giant fibroadenoma. Clin Pract 2011;1:e49 [Crossref] [PubMed]
- Sosin M, Pulcrano M, Feldman ED, et al. Giant juvenile fibroadenoma: a systematic review with diagnostic and treatment recommendations. Gland Surg 2015;4:312-21. [PubMed]
- Dorjgochoo T, Deming SL, Gao YT, et al. History of benign breast disease and risk of breast cancer among women in China: a case-control study. Cancer Causes Control 2008;19:819-28. [Crossref] [PubMed]
- Berkey CS, Tamimi RM, Rosner B, et al. Young women with family history of breast cancer and their risk factors for benign breast disease. Cancer 2012;118:2796-803. [Crossref] [PubMed]
- Nelson ZC, Ray RM, Wu C, et al. Fruit and vegetable intakes are associated with lower risk of breast fibroadenomas in Chinese women. J Nutr 2010;140:1294-301. [Crossref] [PubMed]
- Greenberg R, Skornick Y, Kaplan O. Management of breast fibroadenomas. J Gen Intern Med 1998;13:640-5. [Crossref] [PubMed]
- Eberl MM, Phillips RL Jr, Lamberts H, et al. Characterizing breast symptoms in family practice. Ann Fam Med 2008;6:528-33. [Crossref] [PubMed]
- Quinlan A, O'Brien KK, Galvin R, et al. Quantifying patient preferences for symptomatic breast clinic referral: a decision analysis study. BMJ Open 2018;8:e017286 [Crossref] [PubMed]
- Stachs A, Stubert J, Reimer T, et al. Benign Breast Disease in Women. Dtsch Arztebl Int 2019;116:565-74. [PubMed]
- Hughes LE, Mansel RE, Webster DJ. Aberrations of normal development and involution (ANDI): a new perspective on pathogenesis and nomenclature of benign breast disorders. Lancet 1987;2:1316-9. [Crossref] [PubMed]
- Genova R, Garza RF. Breast Fat Necrosis. StatPearls. Treasure Island (FL), 2020.
- McDonald S, Saslow D, Alciati MH. Performance and reporting of clinical breast examination: a review of the literature. CA Cancer J Clin 2004;54:345-61. [Crossref] [PubMed]
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013;10:742-9.e1-3.
- Harvey JA. Sonography of palpable breast masses. Semin Ultrasound CT MR 2006;27:284-97. [Crossref] [PubMed]
- Namazi A, Adibi A, Haghighi M, et al. An Evaluation of Ultrasound Features of Breast Fibroadenoma. Adv Biomed Res 2017;6:153. [Crossref] [PubMed]
- Duman L, Gezer NS, Balci P, et al. Differentiation between Phyllodes Tumors and Fibroadenomas Based on Mammographic Sonographic and MRI Features. Breast Care (Basel) 2016;11:123-7. [Crossref] [PubMed]
- Cloete DJ, Minne C, Schoub PK, et al. Magnetic resonance imaging of fibroadenoma-like lesions and correlation with Breast Imaging-Reporting and Data System and Kaiser scoring system. SA J Radiol 2018;22:1532. [Crossref] [PubMed]
- Klinger K, Bhimani C, Shames J, et al. Fibroadenoma; from imaging evaluation to treatment. J Am Osteopath Coll Radiol 2019;8:17-30.
- Patterson SK, Neal CH, Jeffries DO, et al. Outcomes of solid palpable masses assessed as BI-RADS 3 or 4A: a retrospective review. Breast Cancer Res Treat 2014;147:311-6. [Crossref] [PubMed]
- Tan BY, Tan PH. A Diagnostic Approach to Fibroepithelial Breast Lesions. Surg Pathol Clin 2018;11:17-42. [Crossref] [PubMed]
- Tse GM, Tan PH. Diagnosing breast lesions by fine needle aspiration cytology or core biopsy: which is better? Breast Cancer Res Treat 2010;123:1-8. [Crossref] [PubMed]
- McLaughlin CM, Gonzalez-Hernandez J, Bennett M, et al. Pediatric breast masses: an argument for observation. J Surg Res 2018;228:247-52. [Crossref] [PubMed]
- Rao R, Ludwig K, Bailey L, et al. Select Choices in Benign Breast Disease: An Initiative of the American Society of Breast Surgeons for the American Board of Internal Medicine Choosing Wisely ® Campaign. Ann Surg Oncol 2018;25:2795-800. [Crossref] [PubMed]
- Harvey JA, Mahoney MC, Newell MS, et al. ACR Appropriateness Criteria Palpable Breast Masses. J Am Coll Radiol 2016;13:e31-e42. [Crossref] [PubMed]
- Dialani V, Chansakul T, Lai KC, et al. Enlarging biopsy-proven fibroadenoma: Is surgical excision necessary? Clin Imaging 2019;57:35-9. [Crossref] [PubMed]
- Hubbard JL, Cagle K, Davis JW, et al. Criteria for excision of suspected fibroadenomas of the breast. Am J Surg 2015;209:297-301. [Crossref] [PubMed]
- Kaufman CS, Littrup PJ, Freman-Gibb LA, et al. Office-based cryoablation of breast fibroadenomas: 12-month followup. J Am Coll Surg 2004;198:914-23. [Crossref] [PubMed]
- Li S, Wu J, Chen K, et al. Clinical outcomes of 1,578 Chinese patients with breast benign diseases after ultrasound-guided vacuum-assisted excision: recurrence and the risk factors. Am J Surg 2013;205:39-44. [Crossref] [PubMed]
- Kovatcheva R, Guglielmina JN, Abehsera M, et al. Ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma-a multicenter experience. J Ther Ultrasound 2015;3:1. [Crossref] [PubMed]
- Neville G, Neill CO, Murphy R, et al. Is excision biopsy of fibroadenomas based solely on size criteria warranted? Breast J 2018;24:981-5. [Crossref] [PubMed]
- Crawford CB, Clay JA, Seydel AS, et al. Surgical Site Infections in Breast Surgery: The Use of Preoperative Antibiotics for Elective, Nonreconstructive Procedures. Int J Breast Cancer 2016;2016:1645192 [Crossref] [PubMed]
- Kopkash K, Clark P. Basic Oncoplastic Surgery for Breast Conservation: Tips and Techniques. Ann Surg Oncol 2018;25:2823-8. [Crossref] [PubMed]
- Jacklin RK, Ridgway PF, Ziprin P, et al. Optimising preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol 2006;59:454-9. [Crossref] [PubMed]
- Hiller A, Lee TJ, Henderson J, et al. Oncoplastic Reduction Pattern Technique Following Removal of Giant Fibroadenoma. Eplasty 2018;18:e4 [PubMed]
- Lee EJ, Chang YW, Oh JH, et al. Breast Lesions in Children and Adolescents: Diagnosis and Management. Korean J Radiol 2018;19:978-91. [Crossref] [PubMed]
Cite this article as: Kopkash K, Yao K. The surgeon’s guide to fibroadenomas. Ann Breast Surg 2020;4:25.

