
Cohesive implants in revisionary breast reconstruction: strategies for optimizing aesthetic outcomes
Introduction
Revisionary alloplastic breast reconstruction is challenging for numerous reasons. The plastic surgeon must manage and favorably alter scar contracture, capsular deformities, as well as the breast envelope. However, one of the most important, and mutable variables is the breast implant itself. Careful pre-operative evaluation as well as meticulous intra-operative assessment and technique are necessary to properly select and utilize the correct implant in each unique clinical circumstance.
Reconstructive breast surgery differs significantly from aesthetic breast surgery in that all or a significant portion of the breast gland is not present. Only the breast-skin envelope or a muscle layer with or without an associated prosthetic or biologic scaffold is left to interface with the implant in prepectoral or submuscular reconstructions, respectively. The overall surgical result will therefore be even more dependent on the characteristics of the underlying implant compared to aesthetic cases in which the breast gland remains to adapt with the prosthesis. In revision-reconstruction cases, the dependence on implant characteristics for overall post-operative breast shape can be even more severe due to a thinning soft tissue envelope, contracture, along with the potential influence of radiation. Selecting the appropriate implant through careful pre-operative assessment and consideration as well as careful implementation of the operative plan are further underscored as critical to a refined result.
Cohesive implants
Available breast implants are broadly generalized into saline and silicone varieties (1,2). While saline implants hold unique advantages, silicone has become the preferred implant fill material for many surgeons due to its more natural weight and feel as well as ability to hold shape (1,2). Silicone implants vary in many characteristics including size, shape, surface texture, and silicone gel cohesiveness, among others, with shell thickness generally in the range of 0.5 millimeters (3). The optimal interplay of these implant properties is dependent on a variety of patient- and surgeon-specific factors that are unique to reconstructive breast surgery (3).
While all silicone implants in the United States today contain a cohesive gel fill of polydimethylsiloxanes (PDMS), there is a wide spectrum of cohesivity that significantly influences breast shape and feel (4). This amount of cohesivity is determined by the degree of crosslinking of the silicone polymers that subsequently increases firmness and cohesivity with increasing crosslinking (5). The three main breast implant manufacturers, Allergan (Dublin, Ireland), Sientra (Santa Barbara, CA, USA), Mentor (Johnson & Johnson, New Brunswick, NJ, USA), all offer cohesive gel implants that have different degrees of cohesivity both within and among different manufacturers (3,6).
Increased gel cross-linking in highly cohesive or form-stable implants creates certain characteristics unique to these implants that must be recognized and utilized appropriately. Increased gel cohesivity yields greater form stability of the implant and subsequently greater resistance to gel deformation (3) as well as a decreased tendency to “leak” silicone in the scenario of shell rupture (7). These implants are therefore firmer and retain their shape better than less cohesive gels. In addition, greater fill volumes in an implant will further increase firmness and retention of shape (8).
Implant properties
Shape and size
Highly cohesive form stable implants are offered in both anatomic and round variants. Anatomic implants are suggested to provide a more natural appearance as their teardrop shape with a tapered upper pole and fuller lower pole mimics the natural slope of the breast. Reported objective comparisons between anatomic and round implants have found no difference in breast augmentation (9,10), though this difference in shape is inherently more influential to the breast contour in reconstructive cases given the absence of overlying parenchyma. That being said, we have anecdotally found the cosmetic outcomes of anatomic implants to be equivalent with cohesive round implants in our practice.
We generally prefer to place round implants in the great majority of reconstructive breast cases, both revisionary and otherwise. Round implants tend to have a more natural feel for the patient, especially when wearing a brassiere. While overall patient satisfaction with shaped and round implants has been demonstrated as similar, shaped implants are reported as feeling firmer (11-13). In our experience, this firmness is significant for patients. Further, any concern for malposition that is present with shaped, textured prostheses is mitigated with round implants.
Even with implant shape as a constant, the remainder of implant variables must be carefully considered in selecting the optimal implant for each patient. In immediate two-stage reconstructive cases, the appropriate size is obvious as guided by the fill of the patient’s tissue expander. Single-stage reconstruction candidates should be those desiring a similar or smaller post-operative breast size compared to pre-operative size (14). Clinical assessment or three-dimensional imaging can assist in predicting pre-operative breast size, which will lead implant size selection in these cases (15,16).
In revisionary cases, the patient’s current implant size will help guide the implant size to be selected in the revision based on patient’s preferences for a smaller or larger size. Ability to place a larger breast implant will be based on the quality of the patient’s breast skin envelope as well as if the breast has been radiated. In non-radiated breast reconstructions, an implant that is approximately 20% greater volume than the current implant can often be placed. This amount will be less in radiated breasts. Conversely, a smaller implant can easily be placed, however skin excision procedures may be required in an immediate or staged fashion based on skin laxity and the amount of volume reduction desired by the patient.
Implant texture
Cohesive breast implants are offered with either smooth or textured surfaces. Textured implants exist along a spectrum of texturing from nanotexture, to microtexture and finally macrotexture based on microscopic classifications of surface area (17,18). The benefits of textured implants include increased stability of the implant in the breast pocket, less risk of malposition and potentially lower risk for capsular contracture (19). If anatomic implants are chosen, texturing is necessary to minimize the possibility of device rotation.
Textured implants, however, have an association with breast implant associated-anaplastic large cell lymphoma (BIA-ALCL). While the exact pathologic mechanism of BIA-ALCL remains to be elucidated, a significant association has been established between the pathogenesis of the disease entity and texturing (20-22). One hypothesis for this correlation is that textured implant surfaces harbor bacteria with resultant biofilm formation inciting a T-cell response leading to BIA-ALCL (23). This is particularly an issue in macrotextured implants (24).
We therefore strongly prefer to utilize smooth surface implants to mitigate risk of BIA-ALCL. The main advantage of textured surfacing is to maintain implant position and avoid malposition, which is obviously a concern in shaped implants (25). However, in utilizing round highly-cohesive implants, malposition is rarely a significant concern rendering any benefit of surface texturing as minimal. Rarely, textured surfacing, even with a round implant, may be advantageous in revision-reconstruction cases. These are generally cases with significant bottoming out of the previous breast implants in patients with a very thin soft tissue envelope. In these circumstances, implant texturing can be helpful in preserving appropriate implant position after the revision when the breast-skin envelope is diminished in its ability to do so. However, this is still rarely, if ever, performed in our practice due to concerns regarding BIA-ALCL. We prefer to utilize scaffold support, generally contour fenestrated acellular dermal matrices, to provide additional positional support rather than surface texturing (26).
Implant projection
Implant projection is an important variable in selecting a patient’s optimal implant, notably in revisionary breast reconstruction cases. Cohesive implants have a wide variety of different projections choices with more projected devices offer a greater anteroposterior dimension for a given implant diameter. Alloplastic breast reconstruction cases differ greatly from aesthetic breast procedures in that most or all of the breast tissue is absent. In reconstruction, the implant fills as well as shapes the final post-operative breast shape. Therefore, as a general rule, more projection is required in reconstructive compared to aesthetic breast cases.
In revision-reconstruction cases, choice of projection can again be guided by the patient’s current implant projection. If more or less projection is desired, the implant type selected by the surgeon and patient should reflect this. The patient’s breast base width should be measured to guide implant diameter selection. The appropriate amount of projection desired is then matched to the estimated diameter in selecting the class of implant projection.
While it is straightforward to decrease projection in revision cases, increasing projection must be done more cautiously as more projection will place increased stress on the overlying breast-skin envelope. Skin flap integrity is not as much of an issue in revision cases as the flaps have been delayed; however, the increased strain can certainly increase tension on wound closure. If not properly selected, an implant that is over-projected for the patient’s skin envelope can result in a flattened anterior appearance as the closure of skin flaps under tension compress the implant. As such, assessing the laxity and quality of the patient’s breast envelope to estimate the volume and dimensions that it can accommodate is paramount. Circumferential capsulotomy can also help to increase the anteroposterior volume to which the breast envelope can adapt.
Implant cohesivity
The cohesivity of implants has important implications for breast form, shape and fill, as well as implant firmness and feel. As discussed previously, all cohesive implants have different levels of cohesivity based on the amount of cross-linking of the silicone gel (27). The degree of implant cohesivity required should be individualized to each patient’s breast morphology and reconstruction goals. As is a theme, reconstructive cases in general will benefit from increased device cohesiveness as there is no overlying breast tissue to camouflage the underlying implant. Any device that can maximize upper pole fullness, maintain device shape, and minimize rippling will be therefore advantageous. Increased implant cohesiveness in revisionary breast reconstruction cases is especially critical in patients with thin breast-skin envelopes. This becomes of even further importance with the increasing trend of placing implants in a prepectoral plane.
These additional variables of firmness and form stability must be considered with regards to the patient’s individual desires as well as the goals of a particular revision procedure. Highly-cohesive, form-stable implants come with greater implant firmness (28), which must be considered with regards to the patients overlying skin envelope and preferences for implant feel. Implant palpability and firmness is inherently more detectable in reconstructive than aesthetic cases and the patients must aware of these implant characteristics preoperatively by seeing and feeling actual implants, particularly with regards to more cohesive implants. These can be considered as potential “drawbacks” for patients, and should be weighed against the potential benefits of increasing cohesivity individually (29). The potential for implant fracture, in which the internal gel fractures but not the outer shell in anatomic, form-stable implants (30), should also be communicated with patients. Proper preoperative education and shared decision-making will greatly help the surgeon and patient to arrive at the most appropriate implant choice based on their tissue characteristics and desired outcomes.
Reconstruction revision
The upper pole
Achieving upper pole fullness can be challenging in breast reconstruction. Contour deformities most commonly present in the upper pole in implant-based breast reconstruction, especially with smooth, round implants as the natural slope of the upper breast is difficult to reconstruct. This becomes even more apparent in prepectoral reconstructions and patients with thin mastectomy flaps as the pectoralis major no longer provides additional upper pole fill as with total submuscular and dual-plane techniques.
Highly cohesive implants provide more stable upper pole fullness as the gel does not descend under the influence of gravity, resulting in improved fill of the upper breast (Figure 1) (31). These implants have had good outcomes in both primary prepectoral reconstruction (32) and revision pocket conversions for animation deformity (33). In revision cases with upper pole hollowing, a form stable option can be used to further augment upper pole volumes to increase superior fullness. Patients with thicker upper pole may benefit from slightly less cohesive options. Whereas those with minimal upper pole thickness do better with highly cohesive, form stable devices (34). Combining cohesive implants with other adjunctive procedures such as capsulorraphy to tighten the pocket, implant size change and fat grafting (35) can be a powerful technique to improve upper pole fullness and contour.
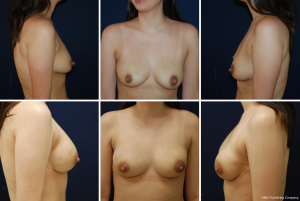
Capsular contracture
Capsular contracture continues to be one of the most common complications after implant-based breast reconstruction and reasons for reconstruction revision (36). While the etiology of capsular contracture is multifactorial, infectious origins have demonstrated a central role in its progression secondary to bacterial contamination and the subsequent stimulation of an inflammatory and fibrotic cascade (37). Prevention relies on minimizing all potential sources of contamination and inflammation (23). The gold standard for treatment of capsular contracture remains total capsulectomy. Given the difficulty of biofilm eradication, implant exchange at the time of capsulectomy is highly advisable. Certain implant characteristics can be considered for exchange to further minimize recurrent contracture.
Textured implants have demonstrated lower rates of capsular contracture (36), particularly in the subglandular plane, but are generally avoided in our practice given their strong association with BIA-ALCL. Highly cohesive implants have also been suggested to have lower rates of capsular contracture secondary to increased firmness resisting contractile forces (38). While improved opposition of mechanical forces may diminish deformity in more severe capsular contracture, these hypotheses still require further research. Other adjunctive treatments include the use of acellular dermal matrix (ADM) as a barrier to the host immune response (26) as well as pocket change, especially from a subglandular to subpectoral plane (Figure 2).
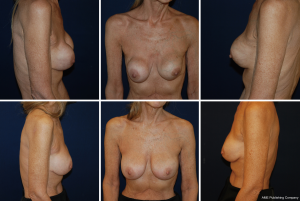
Rippling
Rippling occurs secondary to a deficiency in soft tissue coverage of the implant, particularly in patients with a thinned-out and atrophic soft tissue envelope, as well as excess visible implant deformation. Treatments for rippling are aimed at addressing the soft tissue envelope, the implant itself, or both depending on particular etiology (39). Highly-cohesive implants have decreased wrinkling compared to lower cohesivity implants due to their ability to resist deformation (28). Increased fill in implants similarly aids in decreasing shell deformability and should be taken into consideration when choosing implants for revisions cases with rippling.
When visible implant rippling is a potential or actual concern, changing to a more cohesive implant will help mitigate this issue (Figure 3). In any case, autologous fat grafting is an extremely useful adjunct, along with increasing device cohesiveness, to improve upper pole projection, breast shape, and soft tissue coverage while combating rippling (35,40,41). Additional modifications at the time of revision can also aid in treating wrinkling. Mismatch between the implant and pocket is adjusted with capsulorraphy, and pocket change to a subpectoral pocket, if appropriate, can be considered to provide increased coverage in patients with soft tissue deficiency. Additionally, ADM can be utilized in a similar fashion to reinforce thin overlying tissue (26).
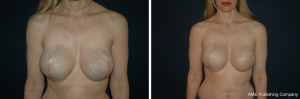
Conclusions
Revisionary alloplastic reconstructive breast surgery requires an understanding and ability to control the complex interplay among numerous factors, perhaps none more important than the breast device itself. It is crucially important that the plastic surgeon be careful in patient assessment to guide implant selection as well as astute and precise in implementing operative strategies to achieve an ideal result. Cohesive implants offer a wide array of differing characteristics to conform to each patient’s individualized needs. Implant size, shape, texturing, projection, and cohesiveness must be thoroughly considered in choosing the optimal implant in each case. Once the device is selected and implemented, the capsule, breast-skin envelope, and/or breast tissue can be managed as necessary with an underlying implant base to provide an outcome with ideal breast shape, size, and symmetry.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Edward I. Chang) for the series “Novel Innovations and Advancements in Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-98). The series “Novel Innovations and Advancements in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. NSK serves as an unpaid editorial board member of Annals of Breast Surgery from Aug 2018 to Jul 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singh N, Picha GJ, Murphy DK. Natrelle silicone breast implant follow-up study: demographics, lifestyle, and surgical characteristics of more than 50,000 augmentation subjects. Plast Reconstr Surg 2016;137:70-81. [Crossref] [PubMed]
- Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J 2010;30:557-70. [Crossref] [PubMed]
- Jewell ML, Bengtson BP, Smither K, et al. Physical properties of silicone gel breast implants. Aesthet Surg J 2019;39:264-75. [Crossref] [PubMed]
- Mohebali K, Wixtrom RN. Breast implant engineering and performance. Plast Reconstr Surg 2018;142:6S-11S. [Crossref] [PubMed]
- Chang EI, Hammond DC. Clinical results on innovation in breast implant design. Plast Reconstr Surg 2018;142:31S-8S. [Crossref] [PubMed]
- Gabriel A, Maxwell GP. The science of cohesivity and elements of form stability. Plast Reconstr Surg 2019;144:7S-12S. [Crossref] [PubMed]
- Calobrace MB, Capizzi PJ. The biology and evolution of cohesive gel and shaped implants. Plast Reconstr Surg 2014;134:6S-11S. [Crossref] [PubMed]
- Jewell ML, Jewell JL. A comparison of outcomes involving highly cohesive, form-stable breast implants from two manufacturers in patients undergoing primary breast augmentation. Aesthet Surg J 2010;30:51-65. [Crossref] [PubMed]
- Hidalgo DA, Weinstein AL. Intraoperative comparison of anatomical versus round implants in breast augmentation: a randomized controlled trial. Plast Reconstr Surg 2017;139:587-96. [Crossref] [PubMed]
- Friedman T, Davidovitch N, Scheflan M. Comparative double blind clinical study on round versus shaped cohesive gel implants. Aesthet Surg J 2006;26:530-6. [Crossref] [PubMed]
- Khavanin N, Clemens MW, Pusic AL, et al. Shaped versus round implants in breast reconstruction: a multi-institutional comparison of surgical and patient-reported outcomes. Plast Reconstr Surg 2017;139:1063-70. [Crossref] [PubMed]
- Macadam SA, Ho AL, Lennox PA, et al. Patient-reported satisfaction and health-related quality of life following breast reconstruction: a comparison of shaped cohesive gel and round cohesive gel implant recipients. Plast Reconstr Surg 2013;131:431-41. [Crossref] [PubMed]
- Nahabedian MY. Shaped versus round implants for breast reconstruction: indications and outcomes. Plast Reconstr Surg Glob Open 2014;2:e116 [Crossref] [PubMed]
- Choi M, Frey JD, Alperovich M, et al. "Breast in a Day": examining single-stage immediate, permanent implant reconstruction in nipple-sparing mastectomy. Plast Reconstr Surg 2016;138:184e-91e. [Crossref] [PubMed]
- Chang JB, Small KH, Choi M, et al. Three-dimensional surface imaging in plastic surgery: foundation, practical applications, and beyond. Plast Reconstr Surg 2015;135:1295-304. [Crossref] [PubMed]
- Tepper OM, Small KH, Unger JG, et al. 3D analysis of breast augmentation defines operative changes and their relationship to implant dimensions. Ann Plast Surg 2009;62:570-5. [Crossref] [PubMed]
- Maxwell GP, Scheflan M, Spear S, et al. Benefits and limitations of macrotextured breast implants and consensus recommendations for optimizing their effectiveness. Aesthet Surg J 2014;34:876-81. [Crossref] [PubMed]
- Atlan M, Nuti G, Wang H, et al. Breast implant surface texture impacts host tissue response. J Mech Behav Biomed Mater 2018;88:377-85. [Crossref] [PubMed]
- Jewell ML, Fickas B, Jewell H, et al. Implant surface options and biofilm mitigation strategies. Plast Reconstr Surg. 2019;144:13S-20S. [Crossref] [PubMed]
- Clemens MW, Brody GS, Mahabir RC, et al. How to diagnose and treat breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg 2018;141:586e-99e. [Crossref] [PubMed]
- Doren EL, Miranda RN, Selber JC, et al. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg 2017;139:1042-50. [Crossref] [PubMed]
- Rastogi P, Riordan E, Moon D, et al. Theories of etiopathogenesis of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg 2019;143:23S-9S. [Crossref] [PubMed]
- Adams WP Jr, Culbertson EJ, Deva AK, et al. Macrotextured breast implants with defined steps to minimize bacterial contamination around the device: experience in 42,000 implants. Plast Reconstr Surg 2017;140:427-31. [Crossref] [PubMed]
- James GA, Boegli L, Hancock J, et al. Bacterial adhesion and biofilm formation on textured breast implant shell materials. Aesthetic Plast Surg 2019;43:490-7. [Crossref] [PubMed]
- Brown MH, Shenker R, Silver SA. Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg 2005;116:768-79; discussion 780-1. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Acellular dermal matrix for reoperative breast augmentation. Plast Reconstr Surg 2014;134:932-8. [Crossref] [PubMed]
- Natrelle. Allergan 2018. Available online: https://www.natrellesurgeon.com/implants
- Panettiere P, Marchetti L, Accorsi D. Soft cohesive silicone gel breast prostheses: a comparative prospective study of aesthetic results versus lower cohesivity silicone gel prostheses. J Plast Reconstr Aesthet Surg 2007;60:482-9. [Crossref] [PubMed]
- Ram E, Lavee J, Freimark D, et al. Improved long-term outcomes after heart transplantation utilizing donors with a traumatic mode of brain death. J Cardiothorac Surg 2019;14:138. [Crossref] [PubMed]
- Calobrace MB. The design and engineering of the MemoryShape breast implant. Plast Reconstr Surg 2014;134:10S-5S. [Crossref] [PubMed]
- Schwartz MR, Haws MJ, Phillips G. Results of the postmarket clinical study of the Sientra 207 highly cohesive gel breast implants in primary and revision augmentation. Plast Reconstr Surg 2018;141:40S-8S. [Crossref] [PubMed]
- Sbitany H, Lee KR. Optimizing outcomes in 2-stage prepectoral breast reconstruction utilizing round form-stable implants. Plast Reconstr Surg 2019;144:43S-50S. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral revision breast reconstruction for treatment of implant-associated animation deformity: a review of 102 reconstructions. Aesthet Surg J. 2018;38:519-26. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Gabriel A. Outcomes utilizing inspira implants in revisionary reconstructive surgery. Plast Reconstr Surg 2019;144:66S-72S. [Crossref] [PubMed]
- Maxwell GP, Gabriel A. Bioengineered breast: concept, technique, and preliminary results. Plast Reconstr Surg 2016;137:415-21. [Crossref] [PubMed]
- Stevens WG, Calobrace MB, Alizadeh K, et al. Ten-year core study data for Sientra's Food and Drug Administration-approved round and shaped breast implants with cohesive silicone gel. Plast Reconstr Surg 2018;141:7S-19S. [Crossref] [PubMed]
- Adams WP Jr. Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg 2009;36:119-26. vii. [Crossref] [PubMed]
- Schwartz MR. Discussion: risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg 2013;132:1126-7. [Crossref] [PubMed]
- Pantelides NM, Srinivasan JR. Rippling following breast augmentation or reconstruction: aetiology, emerging treatment options and a novel classification of severity. Aesthetic Plast Surg 2018;42:980-5. [Crossref] [PubMed]
- Cogliandro A, Barone M, Tenna S, et al. The role of lipofilling after breast reconstruction: evaluation of outcomes and patient satisfaction with BREAST-Q. Aesthetic Plast Surg 2017;41:1325-31. [Crossref] [PubMed]
- Kanchwala SK, Glatt BS, Conant EF, et al. Autologous fat grafting to the reconstructed breast: the management of acquired contour deformities. Plast Reconstr Surg 2009;124:409-18. [Crossref] [PubMed]
Cite this article as: Salibian AA, Karp NS. Cohesive implants in revisionary breast reconstruction: strategies for optimizing aesthetic outcomes. Ann Breast Surg 2020;4:23.

