
Clinical utility of routine parathyroid hormone measurement in women commenced on endocrine therapy for hormone sensitive breast cancer
Introduction
Adjuvant endocrine therapy has been shown to improve oncological outcomes in women with oestrogen receptor (ER)-positive early breast cancer. In recent years, aromatase inhibitors (AIs) (1,2) have largely replaced tamoxifen as the gold standard adjuvant treatment of choice in post-menopausal women with breast cancer, resulting in a near-complete deprivation of oestrogen.
AIs predispose women to accelerated bone mineral density (BMD) loss induced by oestrogen depletion, resulting in a 2-fold increased fracture risk compared to those with physiological post-menopausal bone loss (3). In the event of extended adjuvant AI treatment, there is a marked incidence of osteoporosis even with the use of antiresorptive agents such as bisphosphonates (4). It is important that patients with early breast cancer being considered for endocrine therapy receive appropriate evaluation of bone health. Detection, prevention, and treatment of bone loss in postmenopausal patients with breast cancer has been extensively studied. We have previously reported that baseline vitamin D and parathyroid hormone (PTH) measurement in patients with breast cancer, identifies secondary causes of low BMD in a substantial proportion of patients (5).
Several guidelines exist for both the evaluation and management of bone health for women with early breast cancer, including the National Osteoporosis Foundation (6), the American Society of Clinical Oncology (ASCO) guidelines (7), European Menopause and Andropause Society (EMAS) position statement (8), European Society for Medical Oncology (ESMO) guidelines (9), European Panel guidelines (10), joint position statement of the IOF/CABS/ECTS/IEG/ESCEO/IMS/SIOG (11) and the National Comprehensive Cancer Network (NCCN) Task Force Report (12). Of these, the EMAS and ESMO guidelines suggest the use of PTH in evaluating fracture risk assessment (FRAX). Baseline PTH assessment, and indeed vitamin D, is not recommended in the NCCN guidelines, with the World Health Organisation FRAX tool being favoured instead. All these guidelines suggest the use of a baseline dual-energy X-ray absorptiometry (DXA) scan, and deem the T-score at which antiresorptive therapy should be initiated to be <–2.0. There is uncertainty regarding the duration of antiresorptive treatment during endocrine therapy.
The variation in these guidelines suggest there is still a gap in the evidence relating to the work-up of patients with breast cancer due to start an AI. In this retrospective study, we aimed to correlate serum PTH levels with baseline bone health, and evaluate its clinical utility in predicting individuals on endocrine therapy who are at increased risk of AI-associated bone loss compared to the standard variables of baseline BMD, calcium and vitamin D.
We present the following article in accordance with the STROBE reporting checklist (13) (available at http://dx.doi.org/10.21037/abs-20-15).
Methods
Study design and patient cohort
This retrospective analysis and subsequent manuscript preparation is performed as closely as possible to the STROBE checklist. Women diagnosed with non-metastatic hormone receptor-positive breast cancer and started on endocrine therapy between August 2007 and June 2012 at the Royal Melbourne Hospital, Melbourne, Australia and from the private practice of the service director (GBM) were evaluated. Inclusion criteria were age of 50 years or more at the time of diagnosis, DXA scan and pathology test results available at baseline (within 6 months of starting endocrine therapy), and undergoing at least one follow-up DXA scan whilst on endocrine treatment. Patients in the AI group were taking either anastrozole or letrozole. Patients’ laboratory data were obtained from electronic medical records. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved to be conducted as a quality assurance project under the guidelines of the Human Research Ethics Committee (HREC) of the Royal Melbourne Hospital. Informed consent was waived, as the aim of the project was to review outcomes of treatment to inform future practice, and all information will be protected.
Biochemical assays and imaging
Serum calcium, albumin, PTH and 25-hydroxyvitamin D (vitamin D) were collected. Corrected calcium was calculated. The blood tests closest to initiation of endocrine therapy were utilized in analysis. Similarly, to enable assessment of changes in BMD, the DXA scan completed closest in time to initiation of endocrine therapy was considered the baseline scan and had to be within 6 months of starting endocrine therapy. The last DXA scan completed prior to ceasing endocrine therapy was considered as the final follow-up DXA. In keeping with previous work (5), the femoral neck T-score (FnTs) was used as a marker of overall BMD. Given the varying lengths of time between baseline and follow-up DXA among cohort patients, a standardised approach to assess change in BMD was used by calculating the change in FnTs (ΔFnTs) per year was calculated [(FnTsfinal – FnTsbaseline)/years]. Similarly, a percentage change in total hip BMD was calculated, for comparative purposes to published literature. Baseline FnTs was used to stratify patients as normal (>–1), osteopaenic (≤–1 to >–2.5) and osteoporotic (≤–2.5). Data on calcium/vitamin D/anti-resorptive medication use was not available.
Statistical analysis
Statistical analyses were performed using SPSS (v20; SPSS, Chicago, IL, USA). Linear regression analyses were utilised to determine the association between two or more continuous variables, and a t-test/ANOVA for normally-distributed continuous data against categorical grouping variables. For all analyses, the significant level was set at P<0.05. Data are presented as mean; 95% CI of the mean.
Results
Patient demographics
One hundred and thirty-nine patients were included. Twenty-six patients were prescribed tamoxifen and 108 were prescribed an AI (data missing on 5 patients). Mean age was 65 years; 56–68, and mean time between baseline and final DXA was 3.5 years; 3.0–4.4. Sixty-two patients (46.3%) had osteopaenia, and 12 patients (9.0%) had osteoporosis at baseline.
Baseline PTH measurements identify underlying secondary causes of low BMD
Routine baseline PTH measurements identified four patients (3%) with secondary causes of low BMD requiring surgical management; three patients with a parathyroid adenoma and one patient with parathyroid hyperplasia (see Table 1).
Table 1
| Case | Age | Baseline PTH | Baseline CorrCa | Bone health status | Surgical procedure | Histology | Post-operative PTH |
|---|---|---|---|---|---|---|---|
| 1 | 71 | 16.7 | 3.07 | Osteopaenia | Minimally invasive parathyroidectomy | Parathyroid adenoma | 2.0 |
| 2 | 75 | 9.3 | 2.39 | Osteopaenia | Bilateral neck exploration | Parathyroid adenoma | 3.5 |
| 3 | 79 | 6.4 | 2.57 | Osteoporosis | Bilateral neck exploration | Parathyroid adenoma | 4.1 |
| 4 | 71 | 7.7 | 2.63 | Osteoporosis | Bilateral neck exploration | Parathyroid hyperplasia | 1.8 |
BMD, bone mineral density; PTH, parathyroid hormone.
Baseline PTH, but not vitamin D and corrected calcium, correlates with baseline BMD
The results of a linear regression analysis of the relationship of age at diagnosis, baseline corrected calcium, PTH levels, vitamin D levels and baseline FnTs are summarised in Table 2. There was a significant association identified between baseline FnTs and baseline PTH, but not age, baseline vitamin D and baseline calcium (see Table 2 and Figure 1). When those patients with identified parathyroid disease were removed from analysis, a baseline PTH was no longer significantly associated with baseline FnTs (P=0.201).
Table 2
| Variables | Univariate analysis (95% CI) | P | ||
|---|---|---|---|---|
| Coefficient | Lower bound | Upper bound | ||
| Age at diagnosis | –0.025 | –0.051 | 0.001 | 0.061 |
| PTH at baseline | –0.057 | –0.112 | –0.002 | 0.043 |
| Vitamin D at baseline | –0.002 | –0.011 | 0.007 | 0.607 |
| Corrected calcium at baseline | –0.397 | –2.310 | 1.516 | 0.682 |
FnTs, femoral neck T-score; PTH, parathyroid hormone.
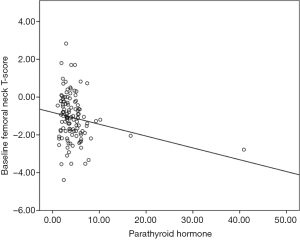
Modality of endocrine therapy, and baseline BMD, but not baseline PTH and vitamin D status, predicts change in BMD
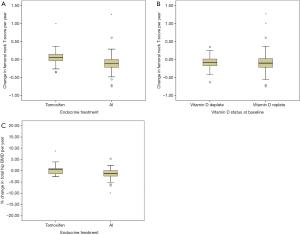
When stratified according to modality of endocrine treatment, there was a net gain in ΔFnTs in patients taking tamoxifen (+0.08; –0.33 to +0.19) compared to net loss in those taking an AI (–0.11; –0.16 to –0.07) (P<0.001) (see Figure 2A). Patients who were vitamin D deplete (n=47) had a net loss in ΔFnTs per year of –0.09; –0.14 to –0.04, similar to those patients that were vitamin D replete (n=85) –0.08; –0.14 to –0.01 (P>0.05) (see Figure 2B). Percentage change in total hip BMD was also calculated, with a net gain for patients on tamoxifen (8.06%; –8.17 to +24.2) vs. loss in patients on AI (–2.23%; –4.11 to –0.34) (P>0.05) (see Figure 2C).
We compared the changes in BMD based on the baseline BMD, as per the baseline FnTs (normal >–1, osteopaenic ≤–1 to >–2.5 and osteoporotic ≤–2.5). Irrespective of modality of endocrine treatment, patients with normal baseline FnTs (n=58) had a higher decrease in ΔFnTs per year of –0.15; –0.20 to –0.10, compared to patients who were osteopaenic at baseline (n=62); –0.03; –0.11 to +0.05, and osteoporotic at baseline (n=12); –0.01; –0.16 to +0.14 (P=0.02) (see Figure 3A). Hence, patients with normal FnTs as baseline had a significantly higher rate of loss of femoral neck bone density. Data on calcium/vitamin D/anti-resorptive medication used by these patients was not available, and hence not analysed as a confounding variable. Indeed there is evidence that both calcium and vitamin D supplementation does not have a considerable effect on BMD, and therefore this was not pursued (14).

The analysis was repeated excluding patients with identified parathyroid disease at baseline and those taking tamoxifen. Similar results were noted (see Figure 3B); patients with normal baseline FnTs (n=48) had a higher decrease in ΔFnTs per year of –0.16; –0.21 to –0.11, compared to patients who were osteopaenic at baseline (n=48); –0.08; –0.16 to +0.01, and osteoporotic at baseline (n=6); –0.07; –0.41 to +0.28 (P=0.243).
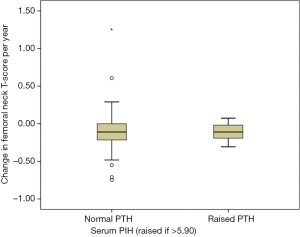
We hypothesized that baseline PTH might identify a group of patients at risk of significant BMD loss while taking AI. However, in patients taking AI and excluding those with an identified parathyroid pathology, the baseline PTH did not predict ΔFnTs. Patients with normal PTH (<5.90, n=78) had a ΔFnTs of –0.11; –0.17 to +0.05, compared to those that had a high PTH (n=12); –0.11; –0.18 to –0.04 (see Figure 4) (P=0.994). Linear regression analysis showed no significant association between baseline PTH and ΔFnTs (data not shown). This was the same for percentage change in total hip BMD (data not shown).
Conclusions
In a previous study (5), we have demonstrated that measurement of PTH at baseline is useful in identifying secondary causes of low BMD. Age and PTH were associated with low baseline BMD. In the current study, it was hypothesized that the baseline PTH may also predict BMD loss over time, with bones possibly being more susceptible to AI-induced loss of BMD in the presence of higher PTH. We confirmed the previous findings that PTH correlates with baseline BMD, but contrary to the hypothesis, did not predict the change in BMD over time. In this cohort, baseline PTH measurement has led to the detection of 4 patients (3%) with disease that required further evaluation and surgical management. Overall, there was a gain in BMD in patients on Tamoxifen, in keeping with the published literature (15). Loss of total hip BMD in patients on AI in this series was 2% per year, which is similar to that observed in the bone sub-study of the ATAC trial, where total hip BMD declined by 7.2% after 5 years of AI treatment. The authors recognize the limitations of the dataset, including the retrospective nature of the study, and small sample size form a single institution.
Prevention and management of bone density loss and subsequent fracture risk is an essential aspect of breast cancer care. Various guidelines exist (6-12), and DXA scan is generally recommended at baseline. The laboratory evaluation of serum calcium and 25-hydroxyvitamin D is recommended prior to commencement of endocrine therapy, with supplementation and further testing often required when abnormalities are detected. However, the measurement of PTH is not included in management guidelines and the usefulness of measuring PTH is unknown.
The value of baseline 25-hydroxyvitamin D measurement prior to commencement of endocrine therapy may be challenged. There is a 25-hydroxyvitamin D deficiency state that leads to osteomalacia. However, studies exploring the associations between 25-hydroxyvitamin D and BMD have led to differing 25-hydroxyvitamin D thresholds at which BMD and bone microstructure changes become apparent (16). Hence, uncertainty remains as to the threshold level of 25-hydroxyvitamin D—and duration of exposure below that threshold—that produces effects on BMD. In this study, we have demonstrated that baseline 25-hydroxyvitamin D does not correlate with baseline BMD. Baseline PTH rather than 25-hydroxyvitamin D may be a more useful marker of underlying bone disease.
An interesting finding is that of an inverse relationship between baseline BMD and loss of BMD over time. Those patients that had normal BMD were more likely to have BMD loss than those who were osteoporotic at baseline (defined by a T-score of >2.5). The confounding influence of treatment of low BMD needs to be considered here—however, as data on calcium/vitamin D/anti-resorptive medication used by these patients was not available, this cannot be accurately ascertained. However, unit protocol is to treat patients with osteoporosis at baseline with anti-resorptive therapy, and to consider it for those with osteopenia, but not for those with a normal BMD at baseline. In this group of patients, appropriate use of bone-protective agents resulted in preservation of BMD that was superior to those patients with a normal BMD at baseline. In addition, it can be concluded that it is important to address bone health in breast cancer survivors on an AI even if the baseline DXA is normal.
The advice for bone protection for patients with normal BMD commencing an AI includes pharmacological and non-pharmacological interventions. Whilst there is evidence of modest improvements in BMD in breast cancer survivors following combined impact and resistance training (17) (as well as the emerging overall benefits of exercise in breast cancer survivors), there is little evidence with regards to calcium supplementation in breast cancer survivors. Current guidelines recommend the use of calcium and vitamin D supplementation to prevent BMD loss for patients starting endocrine therapy for breast cancer, despite the lack of evidence. However, the evidence for prevention of endocrine therapy-induced bone loss with bisphosphonates is consistent, with agents such as zoledronic acid and denosumab (anti-RANK ligand antibody) resulting in positive outcomes (18,19). The National Osteoporosis Foundation (6), and the ASCO guidelines (7) recommend that women with a T-score ≤–2.5 should commence antiresorptive therapy unless contraindicated. It is protocol in our unit to adhere to these guidelines, and although this information was not available to us in our database, it is likely to explain the modest AI-induced BMD loss in patients who were osteoporotic at baseline. Hence, our results show that baseline osteoporosis should not be an absolute contra-indication to AI therapy, as long as appropriate bone protection is prescribed.
Routine measurement of serum PTH on initiation of endocrine therapy may provide information regarding baseline bone health and detect previously undiagnosed parathyroid disease. It is not, however, predictive of future bone loss. Even if the baseline DXA reveals normal BMD, consider bone protective agents, both for bone protection and as adjuvant therapy in post-menopausal patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-15
Data Sharing Statement: Available at http://dx.doi.org/10.21037/abs-20-15
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-15). Prof. GBM serves as an unpaid editorial board member of Annals of Breast Surgery from August 2018 to July 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved to be conducted as a quality assurance project under the guidelines of the Human Research Ethics Committee (HREC) of the Royal Melbourne Hospital. Informed consent was waived, as the aim of the project was to review outcomes of treatment to inform future practice, and all information will be protected.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010;11:1135-41. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341-52. [Crossref] [PubMed]
- Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 2008;26:1051-7. [Crossref] [PubMed]
- Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209-19. [Crossref] [PubMed]
- Mann GB, Kang YC, Brand C, et al. Secondary causes of low bone mass in patients with breast cancer: a need for greater vigilance. J Clin Oncol 2009;27:3605-10. [Crossref] [PubMed]
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25:2359-81. [Crossref] [PubMed]
- Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 2003;21:4042-57. [Crossref] [PubMed]
- Trémollieres FA, Ceausu I, Depypere H, et al. Osteoporosis management in patients with breast cancer: EMAS position statement. Maturitas 2017;95:65-71. [Crossref] [PubMed]
- Coleman R, Body JJ, Aapro M, et al. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2014;25:iii124-37. [Crossref] [PubMed]
- Hadji P, Coleman RE, Wilson C, et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Ann Oncol 2016;27:379-90. [Crossref] [PubMed]
- Hadji P, Aapro MS, Body JJ, et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol 2017;7:1-12. [Crossref] [PubMed]
- Gralow JR, Biermann JS, Farooki A, et al. NCCN task force report: bone health in cancer care. J Natl Compr Canc Netw 2013;11 Suppl 3:S1-50; quiz S51.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296 [Crossref] [PubMed]
- Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet 2014;383:146-55. [Crossref] [PubMed]
- Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 1992;326:852-6. [Crossref] [PubMed]
- Shah S, Chiang C, Sikaris K, et al. Serum 25-hydroxyvitamin d insufficiency in search of a bone disease. J Clin Endocrinol Metab 2017;102:2321-8. [Crossref] [PubMed]
- Winters-Stone KM, Dobek J, Nail LM, et al. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int 2013;24:1637-46. [Crossref] [PubMed]
- Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017;35:2062-81. [Crossref] [PubMed]
- Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015;386:433-43. [Crossref] [PubMed]
Cite this article as: Dave RV, Wong J, Giang A, Kang YC, Le T, Miller JA, Mann GB. Clinical utility of routine parathyroid hormone measurement in women commenced on endocrine therapy for hormone sensitive breast cancer. Ann Breast Surg 2020;4:14.

