Autologous breast reconstruction beyond the DIEP: a narrative review of autologous breast reconstruction options beyond the DIEP flap
Introduction
Although the overwhelming majority of breast reconstructions performed in the United States are implant-based, autologous breast reconstruction is considered the gold standard by many reconstructive plastic surgeons (1-4). The use of the patient’s own tissue has distinct advantages over implants including a more natural feel and appearance, and a more durable reconstruction without concerns regarding device failure, capsular contracture, and the need to exchange the implants during the patient’s lifetime (1-3,5).
Regarding the abdominal donor site, a flap based on the deep inferior epigastric vessels is preferable to a pedicle flap based on the superior epigastric vessels which has been associated with higher fat necrosis rates as well as higher donor site morbidity (1,3).
With the increased comfort in perforator flaps and perforator dissection, as well as improved outcomes in microvascular techniques, the use of microvascular free flaps has revolutionized the field of breast reconstruction (3,6,7). At most high-volume institutions, success rates in free flap breast reconstruction exceed 95% (7). Given these outcomes, the contraindications continue to dwindle while patients previously deemed as unsuitable candidates are now able to receive autologous reconstruction safely and successfully (7). However, in certain circumstances, the abdominal donor site may not be usable due to prior abdominal surgery, body habitus, or prior flap harvest (6,7). In these situations, modifications to the abdominal donor site and alternate donor sites have emerged as viable options for free flap breast reconstruction (6,7).
The present review aims to provide a thorough synopsis of available donor sites in autologous breast reconstruction beyond the traditional deep inferior epigastric perforator (DIEP) flap with a focus on considerations of each donor site to guide reconstructive surgeons in counseling patients interested in autologous breast reconstruction.
Over the years, the main concerns in selecting an alternative flap for breast reconstruction were the donor site morbidity, the shape of the body, the difficulty of the flap’s harvest technique and the possibility of reconstructing a sensate breast. After a thorough analysis of the literature data, this review can also help plastic surgeons in following an algorithm for choosing the appropriate free flap for each patient. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/abs-20-66).
Methods
In writing this review article, we used the PubMed and google scholar sources, by searching for the following keywords: free flap breast reconstruction, autologous breast reconstruction, alternative free flaps for breast reconstruction, nonabdominally-based flaps for breast reconstruction, innovations in breast reconstruction. We analyzed articles published in English, mainly in the last 20 years, reviews or original articles, that best described harvest techniques, complications, tips and tricks for a safe harvest, management of donor site morbidity.
Preoperative evaluation and history
For any patient undergoing surgery, a thorough history and physical is warranted, but in terms of microvascular reconstruction, consideration should be given not only towards the initial reconstruction, but also for the possibility of reoperation in the setting of compromised perfusion to the free flap (3,7). For these reasons, during the history, patients should be asked regarding prior thrombotic events or multiple unplanned miscarriages or spontaneous abortions which may be indicative or a hypercoagulable condition (8). Numerous factors that were previously considered contraindications to free flap breast reconstruction have been largely discredited given the increased experience and knowledge surrounding free tissue transfer (2,7,9).
Nonetheless, patients should be carefully counseled regarding the longer operative and recovery time with autologous reconstruction compared to an implant-based reconstruction (1,4). Comorbidities such as diabetes, hypertension, and smoking have not been associated with increased risks for flap loss, but they can certainly impact wound healing (1,2,5,10,11). Obesity has also long been considered a contraindication to autologous breast; however, again, recent evidence suggests that morbidly obese patients can still undergo microvascular breast reconstruction safely with limited complications and high success rates (11-15). While patients should be encouraged to lose weight, studies examining device-based reconstruction in obese patients have demonstrated significantly higher complication rates, making autologous reconstruction the preferred approach at the authors’ institutions.
Another important consideration for patients interested in undergoing breast reconstruction is the impact of adjuvant therapies (16). Patients who are on hormonal therapy may need to consider discontinuing their tamoxifen for 2 weeks prior to reconstruction and potentially even prolonging the hiatus for an additional 2 weeks following reconstruction (16,17). Again, earlier data had demonstrated increased risks of microvascular thromboses, while more recent studies have not corroborated those findings (16,17). While some studies have demonstrated that radiation therapy has limited detriment to the flap, others have found significant shrinkage and fat necrosis in flaps subjected to radiation which may require additional surgeries and revisions (4,16,18). In general, it is preferable to proceed with definitive reconstruction following completion of radiation (1,4,16,18). One approach is to perform a completely delayed reconstruction following a total mastectomy, or alternatively, a delayed-immediate approach can also be employed by using a tissue expander at the time of the skin-sparing mastectomy (2,4,16,18,19). Following adjuvant radiation to the tissue expander, the authors recommend delaying reconstruction for a minimum of 6 months prior to proceeding with an autologous reconstruction given the increased risks of complications seen when attempting reconstruction earlier. Unfortunately, subjecting patients to multiple operations is a factor that should be discussed and considered when evaluating patients for reconstruction (2,18).
Physical examination and work up
All patients, regardless of the modality of reconstruction, should undergo a thorough physical exam including the breasts as well as the potential donor sites (2). From an oncologic perspective, the breasts should be examined for any palpable masses, skin changes, nipple retraction or discharge, as well as an exam of the axilla for any lymphadenopathy (2). From a plastic surgery perspective, it is important to evaluate the size, symmetry, degree of ptosis, and any prior scars from prior surgeries. The contralateral breast should also be examined both oncologically and for reconstruction, and studies have demonstrated procedures aimed to restore balance and symmetry can be performed safely and simultaneously with the free flap (20,21). Patients should also have appropriate diagnostic imaging of both breasts (19,20). In patients presenting for delayed reconstruction, the pliability and condition of the skin should also be evaluated, particularly in patients who have had prior radiation (2,19).
The physical exam should obviously also consider the available donor sites for autologous reconstruction (2). As the abdominal donor site is the most popular, the abdomen should be examined for scars from prior surgeries (2,22). For patients who have had any type of abdominal surgery, we recommend obtaining a CT angiogram to evaluate the vascular anatomy and perforators preoperatively (22-24). Patients who have had prior liposuction in the abdomen should be counseled regarding the potential risks of fat necrosis in their reconstructed breasts (22,23). Patients who have had a formal abdominoplasty are generally not candidates for an abdominal-based free flap (22).
In this setting, a number of additional donor sites have emerged and been found to be excellent alternatives to the abdomen in autologous breast reconstruction (Table 1) (7). Alternate donor sites that should also be examined are both the superior and inferior gluteal regions, the medial and lateral thigh, and the lower back and flanks (1,3,7). Patients who are not candidates for a DIEP flap may be candidates for a superior gluteal artery perforator (SGAP) or inferior gluteal artery perforator (IGAP) flap (25,26). Or for patients who carry more adiposity in the medial or lateral thigh, the transverse upper gracilis (TUG), profunda artery perforator (PAP) or lateral thigh perforator (LTP) flaps are also reasonable options (7,27,28). Finally, the lumbar artery perforator (LAP) flap is gaining more popularity as a potential donor site for breast reconstruction (29).
Table 1
| Flaps | DIEP | SGAP | IGAP | LTP | PAP | TUG | LAP |
|---|---|---|---|---|---|---|---|
| Dissection difficulty | Low | High | Moderate | Low | Moderate | Low | High |
| Pedicle caliber mismatch | No | Yes | Yes | No | Yes | Yes | Yes |
| Need for position changes | No | Yes | Yes | No | No | No | Yes |
| Sensitivity | Lower intercostal nerves | Superior cluneal nerves | Branches of inferior gluteal nerve (S1–S2) | Lateral femoral cutaneous nerve | Posterior femoral cutaneous nerve | Cutaneous branches of the obturator nerve | Superior cluneal nerves |
DIEP, deep inferior epigastric perforator; SGAP, superior gluteal artery perforator; IGAP, inferior gluteal artery perforator; LTP, lateral thigh perforator; PAP, profunda artery perforator; TUG, transverse upper gracilis; LAP, lumbar artery perforator.
Whether one decides to obtain preoperative imaging is largely at the discretion of the reconstructive surgeon, but studies have demonstrated benefits to preoperative planning where the vascular anatomy and perforators of the donor site are identified using a CT or MR angiogram (30,31). The utility of such imaging studies should be weighed against the added costs but are highly recommended for the novice microsurgeon or when embarking on one of the less commonly performed flaps for the first time (30). In general, we recommend imaging when patients have had prior surgery in the donor site, including liposuction, and for gluteal and lumbar flaps (23,31). For patients undergoing a medial thigh-based flap, preoperative imagine is recommended if the flap is harvested in transverse orientation (30,31).
Imaging of the recipient site is generally only considered in the setting of delayed reconstruction in the radiated chest (32). The authors routinely use the internal mammary vessels as the recipient vessels rather than the thoracodorsal vessels that should be preserved so a pedicled latissimus dorsi myocutaneous flap can still be performed in the setting of a total flap loss. Careful consideration and imaging may be helpful, particularly when one is proceeding with reconstruction of the left breast following a mastectomy and radiation (32). The left internal mammary vein (IMV) is significantly smaller compared to the right side, and an alternate venous outflow may be necessary if the IMV is not usable or diminutive (33). If the IMV is less than 2 mm in size, the authors recommend an alternate recipient vein, such as the cephalic vein which can generally be dissected using stair-step incisions down to the antecubital fossa and reach the medial chest without difficulty (34). Based on basic physic principles (Poiseuille’s law), this is preferable to performing two smaller anastomoses to internal mammary vessels in an antegrade and retrograde fashion.
Donor sites
Abdomen
The abdominal donor site represents the most popular donor site for autologous breast reconstruction for a number of reasons including the ample tissue in terms of both skin and volume that can be harvested with limited donor site morbidity (35). For many patients the post-operative abdominal contour is a secondary benefit to the operation (35,36). Traditionally, one hemiabdomen is utilized to reconstruct a single breast (36). The use of indocyanine green (ICG) has revolutionized the ability to decipher the perfusion of flap and can be beneficial in perforator selection and determining whether the perfusion needs to be augmented. In the setting that the traditional DIEP cannot be performed due to compromised perfusion across the midline, or in the setting of prior surgery, a modification to the traditional DIEP can be performed (22,23). Similarly, for patients who do not have sufficient volume to reconstruct a breast of appropriate size or if patients present for delayed reconstruction in the setting of prior radiation, more skin and volume may be necessary (37,38).
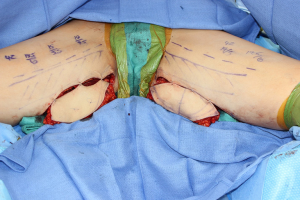
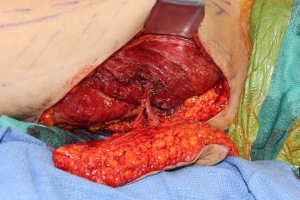
The bipedicle DIEP flap represents a modification to the traditional DIEP flap where the entire abdominal tissue is harvested as a single flap to reconstruct a unilateral breast (Figure 1) (37-42). The authors prefer to leave the flap in continuity to maximize perfusion to the entire flap and also to allow for shaping of the breast with preservation of the skin. Alternatively, the concept of “stacking” flaps has also been increasing in popularity and can be performed with bilateral DIEP flaps as well as with other alternative flaps in order to obtain the necessary volume (36,38,40-42). In this setting, one flap is completely buried to add projection and volume to the reconstructed breast (40-42).
The authors favor using the internal mammary vessels in an antegrade and retrograde fashion. Alternatively, if there are two IMVs of suitable size, both veins can be connected in an antegrade fashion, or if sizable internal mammary perforators are available, they can also serve as the recipient vessels for one of the pedicles of the bipedicle flap (41-43). In general, removal of the cartilage of a single rib is adequate to provide sufficient length to perform both anastomoses if the soft tissue is removed in the 2nd and 3rd intercostal spaces (42,43).
Closure of the donor site should be performed carefully in order to minimize the risks of a bulge or hernia (1,39,44,45). In the setting that both sides are harvested as DIEPs, the fascial should be closed primarily, and plication can correct any pre-existing diastasis and enhance the final abdominal contour (46). However, if more fascia is sacrificed or if a muscle-sparing transverse rectus abdominis myocutaneous (TRAM) was performed, the closure may require supplementation of mesh in order to prevent the risks of complications (1,44).
Buttock
Historically, the gluteal region was the secondary donor site of choice for patients who were not candidates for autologous reconstruction from the abdomen (25,26). Most patients have sufficient soft tissue in the upper or lower buttock to provide ample volume for breast reconstruction (25,26). Both the superior and inferior gluteal arteries have reliable perforators that can supply a generous amount of volume for the SGAP and IGAP flaps respectively (25,47). The location of the perforators can be determined with the use of preoperative imaging, but the location of the main pedicle is well-defined based on anatomic landmarks (31,47,48). A hand-held Doppler can be utilized to confirm the location of perforators to design the flap (mm). Selecting a more lateral perforator is beneficial and will have a longer pedicle which will facilitate the microvascular anastomosis (31,47,48).
Despite the consistent anatomy and availability of tissue in the buttock, there are potential disadvantages that should be considered (47). The gluteal fat tends to have more firmness and is less pliable compared to the other donor sites (25). Another obvious disadvantage of performing either an IGAP or SGAP is the need for a position change unless a sloppy lateral position is used (25,49). While this can save ischemia time, it can be more challenging to perform the dissection for the less experienced surgeon, and generally, harvesting the flap in a prone position is preferred (25). The limited pedicle length, 5 to 8 cm for SGAP and 7 to 10 cm for IGAP, may require a vein graft for the anastomosis which adds another level of complexity for the reconstruction although certainly the anastomosis can be performed safely without the use of vein grafts (25,26,40,50). The intramuscular dissection of the pedicle, the increasing caliber of the vein, around 3 to 4 mm, and the multiple side branches of the artery (“Medusa’s head”) beneath the gluteus maximus muscle, makes the flap elevation and pedicle anastomosis challenging (26,47,48,50). The closure of the donor site is generally well-tolerated but can result in contour deformities and gait disturbances which should be discussed with patients (25,50,51). In patients undergoing an IGAP flap, careful attention should be paid to avoid injury to the posterior femoral cutaneous nerve leading to numbness in the posterior thigh (49,51). If a sensate flap is required, inferior gluteal nerve branches (S1–S2) are preserved and elevated with the flap (25). Ideally, the scars should be concealed taking into account clothing including swimwear, but the scar for the SGAP can be visible, and the scar for the IGAP can migrate and lead to an unsightly scar that is not positioned in the intragluteal crease (25,51,52). The main goal should be a sensate flap because all women desire a functional reconstruction (mm). Considering this, the superior cluneal nerves can be harvested with the SGAP flap (25).
The increased flap loss rates of the gluteal region compared to the abdominal flaps are likely due to a combination of factors including a more challenging anastomosis, perhaps prolonged ischemia time with changes in patient positioning, and potentially traction on the perforators during the dissection for pedicle length (25,47,50).
Thigh
Given the increasing comfort with perforator flaps and understanding of anatomy, the medial thigh has largely replaced the buttock donor site as a secondary flap of choice for autologous breast reconstruction (27,28). The use of the medial thigh often avoids the need for position changes, and harvest of tissue from both thighs can typically provide ample volume for bilateral breast reconstruction in smaller-breasted patients, or can be combined to reconstruct a unilateral mastectomy defect (27,28,53). Alternatively, a thigh-based flap can also be combined with a DIEP flap for bilateral breast reconstruction if more volume is needed than can be provided from either donor site alone (27,53,54).
The two most commonly used flaps from the medial thigh are the TUG and PAP flaps, both of which have reliable anatomy and can be oriented transversely or longitudinally depending on the fat distribution in the medial thigh and patient acceptance of the resulting scar (Figure 2) (55,56). In general, a transversely oriented flap can yield a well-concealed scar but can have the risk of migration and can also distort the vulva while a longitudinal scar can be more visible in clothing (55,57). The TUG incorporates the gracilis muscle and perfusion to the overlying skin is dependent on the presence of a perforator that does not need to be visualized during the dissection (56). The entire muscle is harvested with the flap, but the skin is most reliable proximally where the perforators arise through the bulk of the muscle (55,56). Care should be taken while dissecting the skin island anteriorly not to damage the lymph nodes (27). Furthermore, the cutaneous branches of the obturator nerve can be harvested for a sensate reconstructed breast (7). The vascular pedicle has a 6 to 8 cm length that is considerably shorter and smaller in caliber and can make the microsurgical anastomosis more challenging, often with a significant size mismatch with the internal mammary vessels (56). Although, by including the gracilis muscle, the flap can initially provide enough bulk but tends to shrink over time (56).
The PAP flap has grown noticeably in popularity for breast reconstruction when a DIEP flap cannot be performed or if additional volume is needed to supplement a DIEP flap (53,54,58,59). The flap can also be oriented differently, and the resultant scars are generally well-tolerated with limited donor site morbidity (Figure 3) (53,60). The dissection can be performed in the supine frog-leg position if the flap is harvested longitudinally, or in the lithotomy position if a transversely oriented flap is planned (53,60). Given the reliable perforators arising from the profunda femoris artery, we do not routinely obtain preoperative imaging unless a transversely oriented flap is harvested (53,59). In the setting that the proximal perforator is diminutive or if one is not present, a TUG can be performed if the patient is opposed to the lengthwise scar, or a vertical PAP if the patient is willing to tolerate the scar (60). The pedicle is usually of adequate length, approximately 10 cm, but the caliber of the artery can be smaller than the internal mammary artery (53,58,59). In order to avoid a drastic size mismatch that can lead to turbulent flow, the internal mammary vessels can be dissected more distally, or an internal mammary perforator can be used as well (53). The flap can be raised with posterior femoral cutaneous nerve for improved sensitivity (7).
Anteriorly introduced as septocutaneous tensor fasciae latae (sc-TFL) flap, the newly described LTP flap is based on the septocunateous perforator located between the TFL and the gluteus minimus/medius muscles and originating from the ascending branch of the lateral circumflex femoral artery (LCFA) (29,61). The main advantages of this flap are the fact that usually the lateral thigh region has sufficient fat volume, the flap can be harvested in a supine position, avoiding prolonged ischemia time and the dissection is straightforward, with a consistent and reliable pedicle (61,62). Perforators are located on the horizontal line drawn from the pubic bone that perpendicularly crosses the line from the anterior superior iliac spine (ASIS) to the lateral border of the patella (29). The most cranially and sizeable located perforators should be selected in an attempt to hide the scar in the underwear (29). Unless a sensate reconstruction is wanted, care should be taken to avoid the lateral femoral cutaneous nerve. Flap dissection is made in a suprafascial manner with a 6 to 8 cm pedicle length (29). Refinements have been done over the last years to minimize the donor site morbidity by limiting the flap width, using quilting sutures for closure and improving thigh contour with lipofilling and liposuction (29,62). The LTP flap is an emerging alternative option in breast reconstruction, offering an advantageous pliable tissue with good projection that provides a good aesthetic result (62).
Lumbar
The LAP flap is based on perforators arising at the 3rd or 4th lumbar vertebrae (63-65). While a handheld Doppler can be used reliably to localize the perforators, preoperative imaging may also be useful in helping the microsurgeon design the flap (64-66). Even lean patients do have excess tissue in the lumbar area, and the resulting contour is also quite favorable (67). However, the flap harvest is typically done prone so most microsurgeons using the LAP flap actually have two position changes during surgery (67).
While the consistency of the tissue nicely mimic the abdominal and breast tissue, the pedicle is often considerably shorter, of approximately 6 cm, and many recommend the use of grafts to perform the anastomosis (63,64,67). In fact, many will harvest the deep inferior epigastric vessels in order to lengthen the pedicle (66,67). The dissection becomes quite tedious in order to gain additional length (63). At the transverse process’ region the dissection stops, and clips are avoided after 4 cm pedicle length due to the high risk of nerve roots damage (67). Also, incorporating the superior cluneal nerves to be anastomosed to the intercostal nerves, can make a sensate reconstruction possible (7,66). Lately, the donor area morbidity was significantly decreased with minimal undermining, avoiding overharvesting lumbar tissue, using quilting sutures and vest-over-pants closure of the donor site (64,67).
Early reports using the LAP flap for breast reconstruction have demonstrated high complications and flap loss rates compared to abdominal flaps which may simply be attributed to the learning curve (63,67). Although the cone shape design and the upper pole fullness are important advantages, given the need for vessel grafts, additional position changes, and potential higher risks of flap failure, the LAP flap is a secondary option for autologous breast reconstruction, and serious considerations and discussion with the patient are necessary before committing to this option (64,67).
Monitoring and recovery
While post-operative monitoring and management vary tremendously based on surgeon preference and hospital resources, the majority of thrombotic events tend to occur with the first 24–72 hours following surgery (11). During this time, it is imperative to detect compromised perfusion as early as possible and return to the operating room as expeditiously as possible to maximize the success of flap salvage (11,68-70). Early reports with implantable Dopplers, tissue perfusion monitors, and oxygen saturation monitors have demonstrated promising results, but none supplant the gold standard method—clinical experience, exam, and judgment (68-72). If there is any suspicion for a thrombosis, it is far preferable to have a negative exploration than a total flap loss (68).
All patients, particularly obese patients, should be promptly started on venous thromboembolic event (VTE) prophylaxis; however, whether patients should receive additional anticoagulation is at the surgeon’s discretion (11). Most studies have not demonstrated any protective or preventive benefit using heparin, dextran, or aspirin, although the risks of hematoma are increased (11). For most patients, recovery from the operation is approximately 2–3 months, predominantly due to decreased stamina and energy rather than actual wound healing or pain management (73). With the growing popularity and implementation of enhanced recovery after surgery (ERAS) protocols, pain management has trended away from opioid reliant medications, and more to multi-modality therapy with the aim of decreasing narcotic use and hospital length of stay (73).
Conclusions
While the DIEP flap remains the gold standard of autologous breast reconstruction, in cases without abdominal tissue available, the alternative methods of autologous reconstruction provide good quality tissue coverage. With the description of more donor site options, increased comfort and training with microvascular surgery and perforator flaps, introduction of new technology for flap monitoring, and expanding indications, nearly all patients are candidates for autologous reconstruction.
Considering the reviewed literature data, we believe that we managed to collect enough information to provide an objective and comprehensive review article in order to help plastic surgeons in following a thorough algorithm for choosing the best nonabdominally-based breast reconstructive method, given the particularity of each patient, when the DIEP flap is not available. Given our findings, we think that further research is needed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Edward I. Chang) for the series “Novel Innovations and Advancements in Breast Reconstruction” published in Annals of Breast Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-66
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-66). The series “Novel Innovations and Advancements in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. EIC served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Breast Surgery from Dec 2019 to Nov 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cordeiro PG. Breast reconstruction after surgery for breast cancer. N Engl J Med 2008;359:1590-601. [Crossref] [PubMed]
- Sigurdson L, Lalonde DH. MOC-PSSM CME article: breast reconstruction. Plast Reconstr Surg 2008;121:1-12. [Crossref] [PubMed]
- Healy C, Allen RJ Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg 2014;30:121-25. [PubMed]
- Panchal H, Matros E. Current trends in postmastectomy breast reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Bletsis P, Bucknor A, Chattha A, et al. Evaluation of contralateral and bilateral prophylactic mastectomy and reconstruction outcomes: comparing alloplastic and autologous reconstruction. Ann Plast Surg 2018;80:S144-9. [PubMed]
- Massey MF, Spiegel AJ, Levine JL, et al. Perforator flaps: recent experience, current trends, and future directions based on 3974 microsurgical breast reconstructions. Plast Reconstr Surg 2009;124:737-51. [Crossref] [PubMed]
- Opsomer D, van Landuyt K. Indications and controversies for non-abdominally-based complete autologous tissue breast reconstruction. Clin Plast Surg 2018;45:93-100. [Crossref] [PubMed]
- Bouvier S, Cochery-Nouvellon E, Lavigne-Lissalde G, et al. Comparative incidence of pregnancy outcomes in thrombophilia-positive women from the NOH-APS observational study. Blood 2014;123:414-21. [Crossref] [PubMed]
- Park JE, Chang DW. Advances and innovations in microsurgery. Plast Reconstr Surg 2016;138:915e-24e. [Crossref] [PubMed]
- Torabi R, Stalder MW, Tessler O, et al. Assessing age as a risk factor for complications in autologous breast reconstruction. Plast Reconstr Surg 2018;142:840e-6e. [Crossref] [PubMed]
- Chang EI, Chang EI, Soto-Miranda MA, et al. Comprehensive evaluation of risk factors and management of impending flap loss in 2138 breast free flaps. Ann Plast Surg 2016;77:67-71. [Crossref] [PubMed]
- Fischer JP, Nelson JA, Siemer B, et al. Free tissue transfer in the obese patient: an outcome and cost analysis in 1258 consecutive abdominally based reconstructions. Plast Reconstr Surg 2013;131:681e-92e. [Crossref] [PubMed]
- Jandali S, Nelson JA, Sonnad SS, et al. Breast reconstruction with free tissue transfer from the abdomen in the morbidly obese. Plast Reconstr Surg 2011;127:2206-13. [Crossref] [PubMed]
- Lee KT, Mun GH. Effects of obesity on postoperative complications after breast reconstruction using free muscle-sparing transverse rectus abdominis myocutaneous, deep inferior epigastric perforator, and superficial inferior epigastric artery flap: a systematic review and meta-analysis. Ann Plast Surg 2016;76:576-84. [Crossref] [PubMed]
- Chang EI, Liu J. Prospective evaluation of obese patients undergoing autologous abdominal free flap breast reconstruction. Plast Reconstr Surg 2018;142:120e-25e. [Crossref] [PubMed]
- Kelley BP, Valero V, Yi M, et al. Tamoxifen increases the risk of microvascular flap complications in patients undergoing microvascular breast reconstruction. Plast Reconstr Surg 2012;129:305-14. [Crossref] [PubMed]
- Mirzabeigi MN, Nelson JA, Fischer JP, et al. Tamoxifen (selective estrogen-receptor modulators) and aromatase inhibitors as potential perioperative thrombotic risk factors in free flap breast reconstruction. Plast Reconstr Surg 2015;135:670e-9e. [Crossref] [PubMed]
- Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg 2009;124:395-408. [Crossref] [PubMed]
- Baumann DP, Crosby MA, Selber JC, et al. Optimal timing of delayed free lower abdominal flap breast reconstruction after postmastectomy radiation therapy. Plast Reconstr Surg 2011;127:1100-6. [Crossref] [PubMed]
- Chang EI, Selber JC, Chang EI, et al. Choosing the optimal timing for contralateral symmetry procedures after unilateral free flap breast reconstruction. Ann Plast Surg 2015;74:12-6. [Crossref] [PubMed]
- Chang EI, Lamaris G, Chang DW. Simultaneous contralateral reduction mammoplasty or mastopexy during unilateral free flap breast reconstruction. Ann Plast Surg 2013;71:144-8. [Crossref] [PubMed]
- Roostaeian J, Yoon AP, Sanchez IS, et al. The effect of prior abdominal surgery on abdominally based free flaps in breast reconstruction. Plast Reconstr Surg 2014;133:247e-55e. [Crossref] [PubMed]
- Hamdi M, Khuthaila DK, Van Landuyt K, et al. Double-pedicle abdominal perforator free flaps for unilateral breast reconstruction: new horizons in microsurgical tissue transfer to the breast. J Plast Reconstr Aesthet Surg 2007;60:904-12. [Crossref] [PubMed]
- Wong C, Saint-Cyr M, Arbique G, et al. Three- and four-dimensional computed tomography angiographic studies of commonly used abdominal flaps in breast reconstruction. Plast Reconstr Surg 2009;124:18-27. [Crossref] [PubMed]
- LoTempio MM, Allen RJ. Breast reconstruction with SGAP and IGAP flaps. Plast Reconstr Surg 2010;126:393-401. [Crossref] [PubMed]
- Guerra AB, Metzinger SE, Bidros RS, et al. Breast reconstruction with gluteal artery perforator (GAP) flaps: a critical analysis of 142 cases. Ann Plast Surg 2004;52:118-25. [Crossref] [PubMed]
- Dayan JH, Allen RJ Jr. Lower extremity free flaps for breast reconstruction. Plast Reconstr Surg 2017;140:77S-86S. [Crossref] [PubMed]
- Buntic RF, Horton KM, Brooks D, et al. Transverse upper gracilis flap as an alternative to abdominal tissue breast reconstruction: technique and modifications. Plast Reconstr Surg 2011;128:607e-13e. [Crossref] [PubMed]
- Tuinder SMH, Beugels J, Lataster A, et al. The lateral thigh perforator flap for autologous breast reconstruction: a prospective analysis of 138 flaps. Plast Reconstr Surg 2018;141:257-68. [Crossref] [PubMed]
- Ohkuma R, Mohan R, Baltodano PA, et al. Abdominally based free flap planning in breast reconstruction with computed tomographic angiography: systematic review and meta-analysis. Plast Reconstr Surg 2014;133:483-94. [Crossref] [PubMed]
- Rozen WM, Ting JW, Grinsell D, et al. Superior and inferior gluteal artery perforators: in-vivo anatomical study and planning for breast reconstruction. J Plast Reconstr Aesthet Surg 2011;64:217-25. [Crossref] [PubMed]
- Jugenburg M, Disa JJ, Pusic AL, et al. Impact of radiotherapy on breast reconstruction. Clin Plast Surg 2007;34:29-37. [Crossref] [PubMed]
- Chang EI, Chang EI, Soto-Miranda MA, et al. Demystifying the use of internal mammary vessels as recipient vessels in free flap breast reconstruction. Plast Reconstr Surg 2013;132:763-8. [Crossref] [PubMed]
- Chang EI, Fearmonti RM, Chang DW, et al. Cephalic vein transposition versus vein grafts for venous outflow in free-flap breast reconstruction. Plast Reconstr Surg Glob Open 2014;2:e141 [Crossref] [PubMed]
- Selber JC, Fosnot J, Nelson J, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: Part II. Bilateral reconstruction. Plast Reconstr Surg 2010;126:1438-53. [Crossref] [PubMed]
- Beugels J, Vasile JV, Tuinder SMH, et al. The stacked hemiabdominal extended perforator flap for autologous breast reconstruction. Plast Reconstr Surg 2018;142:1424-34. [Crossref] [PubMed]
- Chang EI, Chang EI, Ito R, et al. Challenging a traditional paradigm: 12-year experience with autologous free flap breast reconstruction for inflammatory breast cancer. Plast Reconstr Surg 2015;135:262e-9e. [Crossref] [PubMed]
- Beahm EK, Walton RL. The efficacy of bilateral lower abdominal free flaps for unilateral breast reconstruction. Plast Reconstr Surg 2007;120:41-54. [Crossref] [PubMed]
- Agarwal JP, Gottlieb LJ. Double pedicle deep inferior epigastric perforator/muscle-sparing TRAM flaps for unilateral breast reconstruction. Ann Plast Surg 2007;58:359-63. [Crossref] [PubMed]
- DellaCroce FJ, Sullivan SK, Trahan C. Stacked deep inferior epigastric perforator flap breast reconstruction: a review of 110 flaps in 55 cases over 3 years. Plast Reconstr Surg 2011;127:1093-99. [Crossref] [PubMed]
- Chang EI, Kronowitz SJ. Dual-pedicle flap for unilateral autologous breast reconstruction revisited: evolution and optimization of Flap design over 15 years. Plast Reconstr Surg 2016;137:1372-80. [Crossref] [PubMed]
- Stalder MW, Lam J, Allen RJ, et al. Using the retrograde internal mammary system for stacked perforator flap breast reconstruction: 71 breast reconstructions in 53 consecutive patients. Plast Reconstr Surg 2016;137:265e-77e. [Crossref] [PubMed]
- Tomioka YK, Uda H, Yoshimura K, et al. Studying the blood pressures of antegrade and retrograde internal mammary vessels: do they really work as recipient vessels? J Plast Reconstr Aesthet Surg 2017;70:1391-6. [Crossref] [PubMed]
- Chang EI, Chang EI, Soto-Miranda MA, et al. Comprehensive analysis of donor-site morbidity in abdominally based free flap breast reconstruction. Plast Reconstr Surg 2013;132:1383-91. [Crossref] [PubMed]
- Wu LC, Bajaj A, Chang DW, et al. Comparison of donor-site morbidity of SIEA, DIEP, and muscle-sparing TRAM flaps for breast reconstruction. Plast Reconstr Surg 2008;122:702-9. [Crossref] [PubMed]
- Uda H, Kamochi H, Sarukawa S, et al. Clinical and quantitative isokinetic comparison of abdominal morbidity and dynamics following DIEP versus muscle-sparing free TRAM flap breast reconstruction. Plast Reconstr Surg 2017;140:1101-9. [Crossref] [PubMed]
- Ahmadzadeh R, Bergeron L, Tang M, et al. The superior and inferior gluteal artery perforator flaps. Plast Reconstr Surg 2007;120:1551-6. [Crossref] [PubMed]
- Allen RJ, Tucker C Jr. Superior gluteal artery perforator free flap for breast reconstruction. Plast Reconstr Surg 1995;95:1207-12. [Crossref] [PubMed]
- Mirzabeigi MN, Au A, Jandali S, et al. Trials and tribulations with the inferior gluteal artery perforator flap in autologous breast reconstruction. Plast Reconstr Surg 2011;128:614e-24e. [Crossref] [PubMed]
- Rad AN, Flores JI, Prucz RB, et al. Clinical experience with the lateral septocutaneous superior gluteal artery perforator flap for autologous breast reconstruction. Microsurgery 2010;30:339-47. [Crossref] [PubMed]
- Allen RJ, Levine JL, Granzow JW. The in-the-crease inferior gluteal artery perforator flap for breast reconstruction. Plast Reconstr Surg 2006;118:333-9. [Crossref] [PubMed]
- Kronowitz SJ. Redesigned gluteal artery perforator flap for breast reconstruction. Plast Reconstr Surg 2008;121:728-34. [Crossref] [PubMed]
- Haddock NT, Gassman A, Cho MJ, et al. 101 consecutive profunda artery perforator flaps in breast reconstruction: lessons learned with our early experience. Plast Reconstr Surg 2017;140:229-39. [Crossref] [PubMed]
- Haddock N, Nagarkar P, Teotia SS. Versatility of the profunda artery perforator flap: creative uses in breast reconstruction. Plast Reconstr Surg 2017;139:606e-12e. [Crossref] [PubMed]
- Park JE, Alkureishi LW, Song DH. TUGs into VUGs and friendly BUGs: transforming the gracilis territory into the best secondary breast reconstructive option. Plast Reconstr Surg 2015;136:447-54. [Crossref] [PubMed]
- Buchel EW, Dalke KR, Hayakawa TE. The transverse upper gracilis flap: efficiencies and design tips. Can J Plast Surg 2013;21:162-6. [Crossref] [PubMed]
- Craggs B, Vanmierlo B, Zeltzer A, et al. Donor-site morbidity following harvest of the transverse myocutaneousgracilis flap for breast reconstruction. Plast Reconstr Surg 2014;134:682e-91e. [Crossref] [PubMed]
- Allen RJ Jr, Lee ZH, Mayo JL, et al. The profunda artery perforator flap experience for breast reconstruction. Plast Reconstr Surg 2016;138:968-75. [Crossref] [PubMed]
- Allen RJ, Haddock NT, Ahn CY, et al. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg 2012;129:16e-23e. [Crossref] [PubMed]
- Hunter JE, Lardi AM, Dower DR, et al. Evolution from the TUG to PAP flap for breast reconstruction: comparison and refinements of technique. J Plast Reconstr Aesthet Surg 2015;68:960-5. [Crossref] [PubMed]
- Tessler O, Guste J, Bartow MJ, et al. Stacked lateral thigh perforator flap as a novel option for autologous breast reconstruction. Plast Reconstr Surg 2019;143:1601-4. [Crossref] [PubMed]
- Haddock NT, Teotia SS. Discussion: the lateral thigh perforator flap for autologous breast reconstruction: a prospective analysis of 138 flaps. Plast Reconstr Surg 2018;141:269-70. [Crossref] [PubMed]
- Peters KT, Blondeel PN, Lobo F, et al. Early experience with the free lumbar artery perforator flap for breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:1112-9. [Crossref] [PubMed]
- Hamdi M, Craggs B, Brussaard C, et al. Lumbar artery perforator flap: an anatomical study using multidetector computed tomographic scan and surgical pearls for breast reconstruction. Plast Reconstr Surg 2016;138:343-52. [Crossref] [PubMed]
- Sommeling CE, Colebunders B, Pardon HE, et al. Lumbar artery perforators: an anatomical study based on computed tomographic angiography imaging. Acta Chir Belg 2017;117:223-26. [Crossref] [PubMed]
- Opsomer D, Vyncke T, Depypere B, et al. Lumbar flap versus the gold standard: comparison to the DIEP flap. Plast Reconstr Surg 2020;145:706e-14e. [Crossref] [PubMed]
- Opsomer D, Stillaert F, Blondeel P, et al. The lumbar artery perforator flap in autologous breast reconstruction: initial experience with 100 Cases. Plast Reconstr Surg 2018;142:1e-8e. [Crossref] [PubMed]
- Chang EI, Ibrahim A, Zhang H, et al. Deciphering the sensitivity and specificity of the implantable doppler probe in free flap monitoring. Plast Reconstr Surg 2016;137:971-6. [Crossref] [PubMed]
- Koolen PG, Vargas CR, Ho OA, et al. Does increased experience with tissue oximetry monitoring in microsurgical breast reconstruction lead to decreased flap loss? the learning effect. Plast Reconstr Surg 2016;137:1093-101. [Crossref] [PubMed]
- Um GT, Chang J, Louie O, et al. Implantable Cook-Swartz doppler probe versus Synovis Flow Coupler for the post-operative monitoring of free flap breast reconstruction. J Plast Reconstr Aesthet Surg 2014;67:960-6. [Crossref] [PubMed]
- Frey JD, Stranix JT, Chiodo MV, et al. Evolution in monitoring of free flap autologous breast reconstruction after nipple-sparing mastectomy: is there a best way? Plast Reconstr Surg 2018;141:1086-93. [Crossref] [PubMed]
- Levine SM, Snider C, Gerald G, et al. Buried flap reconstruction after nipple-sparing mastectomy: advancing toward single-stage breast reconstruction. Plast Reconstr Surg 2013;132:489e-97e. [Crossref] [PubMed]
- Temple-Oberle C, Shea-Budgell MA, Tan M, et al. ERAS society. consensus review of optimal perioperative care in breast reconstruction: enhanced recovery after surgery (ERAS) society recommendations. Plast Reconstr Surg 2017;139:1056e-71e. [Crossref] [PubMed]
Cite this article as: Bordianu A, Leoveanu I, Chang EI. Autologous breast reconstruction beyond the DIEP: a narrative review of autologous breast reconstruction options beyond the DIEP flap. Ann Breast Surg 2020;4:15.