
Use of indocyanine green in detection of breast axillary sentinel lymph nodes
Introduction
Sentinel lymph node biopsy is one of the most important prognostic factors in patients diagnosed with breast cancer. It has evolved over the last few decades in breast cancer management (1). The sentinel lymph nodes (SLNs) are the initial nodes that directly receive lymph drainage from the primary tumor. If the first lymph node draining a tumor is negative for malignant cells, there is a high probability that the remaining lymph nodes in the relevant primary and subsequent basins will also be negative (2). Thus, identification and assessment of SLN provides an accurate clinical window into the regional basin (1).
Sentinel node resection was designed to minimize side effects of lymph node surgery but still offer outcomes equivalent to axillary node dissection (3). Sentinel lymph node biopsy has not been standardized universally, therefore, its method varies by institution and surgeon (4). Technetium-99m sulfur colloid and blue dye (consisting of either isosulfan blue or methylene blue) are commonly used to detect sentinel lymph nodes (3). A metanalysis showed detection rates of 91.9% for blue dye in combination with radiocolloid for detection of sentinel lymph nodes versus 83.1% for blue dye alone and 89% for radiocolloid alone (4). According to the American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early stage breast cancer; the greatest proportion of successful mappings and the lowest false-negative rates were associated with studies in which both blue dye and radiolabeled colloid were used (5).
However, there are potential drawbacks to the use of blue dye for sentinel lymph node localization. Isosulfan blue has been documented causing blue urticaria, edema, and even anaphylaxis (6,7). Methylene blue can cause skin necrosis, induration and erythema (8). In addition, a five-minute breast massage is done after the blue dye is injected to dilate the breast lymphatics, and therefore, assist in visualization of the dye (9). Given these potential side effects and extra operating room time, new techniques in sentinel lymph node localization are actively being studied.
Indocyanine green (ICG) has emerged as a potential addition or even alternative to the blue dye for detecting sentinel lymph nodes. ICG is a near infrared (NIR), water soluble tricarbocyanine fluorescent dye. ICG was first developed by Kodak in 1955 for use in Near Infrared photography. In 1959, the FDA cleared ICG for clinical use, which largely consisted of retinal angiography. ICG works within the tissue optical window whereby wavelengths of both excitation and fluorescence penetrate several millimeters through tissue. It is anionic, relatively hydrophobic, and has increased uptake into the lymphatics (10). Multiple studies have shown a higher detection rate using ICG in comparison to blue dye (11,12).
This study compares Technetium 99m (99mTc), isosulfan blue, and ICG in the detection of axillary sentinel lymph nodes. It evaluates the role of ICG in relation to isosulfan blue and whether ICG provides improved SLN identification and axillary staging. In this study design, each patient receives every method of detection and serves as their own control.
We present the following article/case in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/abs-20-22).
Methods
Patients undergoing Sentinel Lymph Node Biopsy were selected to receive a combination of Isosulfan Blue, Technetium 99m (99mTc), and Indocyanine Green (ICG). 52 total patients were included in this prospective study between June 2017 to October 2018. Patients with biopsy proven breast carcinoma requiring sentinel lymph node biopsy were included. 47 patients had invasive ductal carcinoma, four patients had invasive lobular carcinoma, and one patient had invasive mammary carcinoma. Table 1 illustrates patient data. Patients were excluded from this study if they had allergies to any of the 3 localization methods. This was performed by two surgeons at AMITA Health Saint Joseph Hospital in Chicago, IL. Uniform approach was used. Primary outcome of this study was method of node detection in accordance with node pathology.
Table 1
| Patient data | Outcome |
|---|---|
| Total number of patients | 52 |
| Gender | |
| Female | 51 |
| Male | 1 |
| Average age | 59.5 |
| Average BMI | 28.3 |
| Race | |
| Caucasian | 25 |
| African American | 13 |
| Asian | 8 |
| Hispanic | 6 |
| Cancer type | |
| Invasive ductal | 47 |
| Invasive lobular | 4 |
| Invasive mammary | 1 |
| Receptor status | |
| ER+ | 42 |
| PR+ | 35 |
| Her2+ | 11 |
Patients received 99mTc intradermal injection 2 hours prior to surgery. Prior to skin prep, Isosulfan Blue was injected intradermally followed by a 5-minute massage. The patient was prepped and draped. 2.5 mg/mL concentration ICG was then injected intradermally with 0.3 cc at 12:00 and either 3:00 (left breast) or 9:00 (right breast) positions. Transverse incision was made according to gamma signal detection. The clavipectoral fascia was incised and evaluated for any blue dye uptake. The axilla was also then visualized for ICG uptake of sentinel lymph nodes. (Figure 1) Lymph nodes detected with 99mTc, ICG or that were blue were dissected and removed for pathology (Figure 2). After each node dissection and removal, documentation was made for positivity with either isosulfan blue, 99mTc count, and/or ICG (Figure 3). The axilla was reviewed for any further sentinel lymph nodes via all three methods prior to closure. Number of nodes removed were based on positive findings with each detection method. Final pathology was reviewed and documented for each specific lymph node removed.
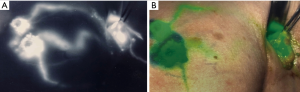

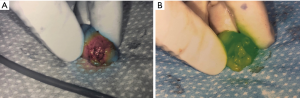
The study was conducted in accordance with the Declaration of Helsinki. Institutional Review Board of Presence Saint Joseph Hospital approved this study (ID #2018-19). Informed consent was obtained.
Results
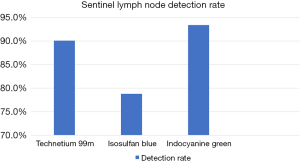
A total of 137 nodes were identified from the 52 patients. Of those 137 nodes, 124 nodes were detected via 99mTc, 108 detected with Isosulfan Blue, and 128 with Indocyanine Green. The detections rates were 90.1% with 99mTc, 78.8% with Isosulfan Blue, and 93.4% with ICG (Figure 4)
An average of 2.63 nodes were identified per patient when including all three detection methods. Nodes detected via 99mTc or Isosulfan Blue had a detection average of 2.51 nodes per patient versus 99mTc or ICG with 2.61 nodes per patient.
Combination of 99mTc and isosulfan blue showed a detection rate of 95.0% versus 99mTc and ICG of 99.3%.
Of those 137 nodes identified, 20 nodes were positive for metastasis in 13 of the 52 patients. Node positivity of 14.6%. In one patient, an extra node with positive disease was identified with ICG that was not identified with either 99mTc or isosulfan blue.
Discussion
This prospective study was designed to assess ICG’s role in sentinel lymph node biopsy in early breast cancer patients and whether it was non-inferior to the blue dye method of localization. Previous studies have shown different variations of using ICG for sentinel lymph node detection.
Our results showed a higher sentinel lymph node detection rate with ICG than with isosulfan blue, 93.4% versus 78.8% respectively. Of all the nodes detected, the percentage total was much more reliably detected with ICG than with isosulfan blue. There have been similar results among studies comparing ICG and blue dye with the addition of favoring the combination of ICG with blue dye (11,12). A prospective cohort study showed a nodal detection rate of 97% for ICG, 89% for methylene blue, and 99.5% for a combination of both (P<0.001). However, that cohort study also highlighted the potential side effects of using blue dye. The study reported 5/82 patients developed permanent tattooing of the skin which was defined as greater than 6 months of skin pigmentation and this was attributed to injection of methylene blue (11).
Due to these potential adverse reactions, ICG could provide a complimentary advantage to radioisotope in the absence of blue dye. This study showed detection rates of 99mTc with either isosulfan blue or ICG to be 95% vs. 99.3% respectively. This highlights the potential similarity or slight improvement in detecting sentinel lymph nodes with ICG and radioisotope only without the added adverse reactions. A multicenter prospective study comparing ICG to radioisotope in 821 patients showed identical detection of sentinel lymph nodes (SLN) using ICG and radioisotope 97.2% vs. 97.0% respectively, (P=0.88). More important and statistically significant was the combination of ICG and radioisotope in the detection of SLN compared to radioisotope alone (99.8 vs. 97.0%, P<0.001). The study also reported no serious adverse events related to ICG hypersensitivity (13).
With recent improvements in imaging technology, there has been a large increase in interest and application of ICG, including use in perfusion assessment, lymph node identification, laparoscopic biliary tree visualization, ureter visualization, etc. (10,14). ICG has been shown to be nontoxic and nonionizing. ICG solution does contain sodium iodide, thus it is not used in patients with iodine allergy (10). An absence in allergic reaction may also be due to interstitial use in lymphatics rather than intravenously, which is more prone to adverse reactions (10,15). There were no immediate adverse reactions during this prospective study.
Another important consideration is the ease and efficiency with ICG. With blue dye, a 5-minute massage was performed after the blue dye was injected to dilate the lymphatics. This assists in the visualization of the dye during dissection to identify the sentinel lymph nodes (9). Another time factor is that the radioisotope was injected 2 hours prior to surgery. Injection less than two hours prior to surgery can affect the detection ability of this particular gamma probe (16).
The trimodal localization performed in this study allowed each patient to serve as their own control and helped identify key benefits and limitations to each method of detection. Our results were detected via 52 patients, and therefore, a larger scale study is needed to confirm our findings. In addition, long term follow-up may have provided further information on adverse reactions to each localization method.
Conclusions
Sentinel lymph node detection with Indocyanine Green is non-inferior to Isosulfan Blue. It shows an improved detection rate alone in comparison to isosulfan blue and in combination with Technetium 99m. In addition, it eliminates the need for a five-minute massage and has the potential of less side effects when compared to blue dye. Larger scale trials with proper follow up will be necessary to confirm the results of this preliminary study.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-22
Data Sharing Statement: Available at http://dx.doi.org/10.21037/abs-20-22
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-22). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board of Presence Saint Joseph Hospital approved this study (ID #2018-19). Informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zahoor S, Haji A, Battoo A, et al. Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update. J Breast Cancer 2017;20:217-27. [Crossref] [PubMed]
- Moncayo VM, Aarsvold JN, Alazraki NP. Lymphoscintigraphy and sentinel nodes. J Nucl Med 2015;56:901-7. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. [Crossref] [PubMed]
- Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer 2006;106:4-16. [Crossref] [PubMed]
- Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005;23:7703-20. [Crossref] [PubMed]
- Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol 2006;13:491-500. [Crossref] [PubMed]
- Raut CP, Hunt KK, Akins JS, et al. Incidence of anaphylactoid reactions to isosulfan blue dye during breast carcinoma lymphatic mapping in patients treated with preoperative prophylaxis: results of a surgical prospective clinical practice protocol. Cancer 2005;104:692-9. [Crossref] [PubMed]
- Bleicher RJ, Kloth DD, Robinson D, et al. Inflammatory cutaneous adverse effects of methylene blue dye injection for lymphatic mapping/sentinel lymphadenectomy. J Surg Oncol 2009;99:356-60. [Crossref] [PubMed]
- James TA, Coffman AR, Chagpar AB, et al. Troubleshooting Sentinel Lymph Node Biopsy in Breast Cancer Surgery. Ann Surg Oncol 2016;23:3459-66. [Crossref] [PubMed]
- Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585 [Crossref] [PubMed]
- Guo J, Yang H, Wang S, et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol 2017;15:196. [Crossref] [PubMed]
- He K, Chi C, Kou D, et al. Comparison between the indocyanine green fluorescence and blue dye methods for sentinel lymph node biopsy using novel fluorescence image-guided resection equipment in different types of hospitals. Transl Res 2016;178:74-80. [Crossref] [PubMed]
- Sugie T, Kinoshita T, Masuda N, et al. Evaluation of the Clinical Utility of the ICG Fluorescence Method Compared with the Radioisotope Method for Sentinel Lymph Node Biopsy in Breast Cancer. Ann Surg Oncol 2016;23:44-50. [Crossref] [PubMed]
- Siddighi S, Yune JJ, Hardesty J. Indocyanine green for intraoperative localization of ureter. Am J Obstet Gynecol 2014;211:436.e431-432. [Crossref] [PubMed]
- Zhai Q, Wang Y, Tian A. Severe hemodynamic instability after indocyanine green injection during off-pump coronary artery bypass grafting: A case report. Medicine (Baltimore) 2017;96:e8766 [Crossref] [PubMed]
- Schneebaum S, Stadler J, Cohen M, et al. Gamma probe-guided sentinel node biopsy--optimal timing for injection. Eur J Surg Oncol 1998;24:515-9. [Crossref] [PubMed]
Cite this article as: Bhakta PS, Farnand AW, Osipova MA, Connolly MM. Use of indocyanine green in detection of breast axillary sentinel lymph nodes. Ann Breast Surg 2020;4:9.

