
Precision surgery for multifocal multicentric breast cancer in the genomic era
Introduction
In the early twentieth century, there was a tendency to overcompensate for a rudimentary understanding of pathology by performing extensive, possibly excessive surgery, as attested by reports in the year 1929, where mastectomies were being performed for entities described as ‘chronic cystic mastitis’ or ‘papillary cystadenoma’, which eventually proved to be benign (1). Advances in pathology allowed definitive diagnosis of malignant disease and appropriate selection of patients for mastectomy, with a concomitant reduction in the extent of surgery for benign conditions. Mastectomy formed the mainstay of treatment for breast cancer until prospective randomised controlled trials (RCTs) in the late twentieth century demonstrated non-inferior survival outcomes with breast conservation treatment (BCT) (2). This process represented the blueprint of de-escalation of surgical therapy, where selection of appropriate candidates was based on clinical parameters, comprising tumor size and palpability of lymph nodes (2,3). A parallel development of interest which occurred with the introduction of BCT was the demonstration of multiple ipsilateral breast malignancies (4,5). Warnings were sounded that the treatment of a clinically unifocal tumor would result in residual foci of cancer, leading to poor local control. However, this initial concern was sufficiently allayed when the results of the National Surgical and Adjuvant Breast and Bowel Project (NSABP) B-06 study were reported, leading to the conclusion that radiotherapy played a significant role in eradicating and controlling subclinical tumor foci (6,7). In the further course of pathologic development, estrogen and progesterone receptors (8), prognostic indices (9), and axillary nodal involvement contributed to therapeutic decisions regarding adjuvant treatment (10).
The discovery of the molecular structure of deoxyribose nucleic acid (DNA) in 1953 by Watson & Crick (11), and the completion of the genome project (12) marked the beginning of the genomic era, making identification of specific tumor mutations possible. Molecular signatures have both prognostic and predictive capabilities for therapeutic intervention. Thus far, genomics have been applied to intensifying or de-escalating medical therapy. There is preliminary data on its use for radiotherapy. However, contemporary evidence on precision surgery in the genomic era is scarce, particularly so for multifocal multicentric breast cancer (MFMCBC). This review therefore seeks to explore the use of genomics in individualizing surgical decisions for MFMCBC.
Methods
A MEDLINE (PubMed) search based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (13) was conducted using a combination of the terms ‘precision’, ‘surgery’, ‘multifocal’, ‘multicentric’, ‘breast’, ‘cancer’ and ‘genomics’. Articles which were not in English, where abstracts only were available and those which did not identify the use of genomics in surgical decision making were excluded. References of relevant articles were scrutinised for pertinent studies. The search was performed in November 2019 and there was no prior time limit set for inclusion of data.
Results
Literature search
There were a total of 509 potential citations from the initial MEDLINE search (Figure 1).
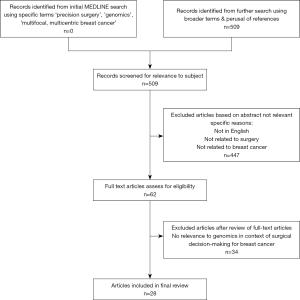
Amongst these, five articles discussed application of molecular profiling in surgical therapeutics. There was a nil return for articles specific to the combination of search terms: ‘genomics’ and ‘surgery’ and ‘multifocal multicentric breast cancer’. Hence, articles were gleaned from additional searches using broader terms. In total, 28 articles discussing relevant issues pertaining to genomics, surgery and MFMCBC were included in this review. It is noteworthy that there were no studies reporting the use of genomics to guide decision-making in terms of surgical therapeutic approach outcomes, nor were there any data on biomarkers for predicting ipsilateral or contralateral breast cancer. Due to the paucity of studies relating to genomics and treatment selection in this clinical setting, a narrative review was performed.
Discussion
Sick lobe hypothesis and MFMCBC
The presence of multiple ipsilateral breast cancer (MIBC) foci was established at the same time period as the initiation of BCT (3-6). It was demonstrated that occult foci of malignancy was detected in 63% of cases thought to be unifocal in nature, with 43% occurring more than 2 cm from the index lesion (6). In this context, caution was sounded against the routine use of BCT. However, this warning was overridden by the results of the NSABP B-06 study, which reported adequate local control with BCT when negative margins were obtained together with whole breast radiotherapy (WBRT) (7). This data, together with evidence from other prospective RCTs, led to the establishment of BCT as the preferred modality of surgical treatment for early breast cancer (14). These guidelines, however, did not extend to clinically detected MFMCBC and as early studies suggested poorer local control with BCT (15,16), initial recommendations favored mastectomy (17).
The ‘sick lobe’ hypothesis was derived through comprehensive study of the complexities involving breast anatomy and pathology (18,19). Detailed analysis of large format histology sections provided ability to differentiate unifocal, multifocal (MF), diffuse and multicentric (MC) disease (19,20). It is postulated that genetic instability and susceptibility originates during embryology, and these aberrations are distributed along the arborization of ducts all along the individual lobe to the terminal lobular ductal units (TDLUs) (20). Further mutagenesis within a single duct or TDLU results in unifocal cancer, while simultaneous carcinogenic changes at several points within the same lobe produced MF disease. Concurrent changes in more than one lobe generated MC tumors. Disease within the same lobe, therefore, would be expected to harbor similar genetic mutations, while it might be predicted that those in different lobes, or true MC disease, will have genomic heterogeneity.
Implications of genomics in classification of MFMCBC
Interestingly, independent of large format histology, a recent study conducted on the genomic characteristics of MF cancer appear to support the sick lobe theory (21). In this study by Desmedt et al., multifocal breast cancer (MFBC) was defined as any ipsilateral, synchronous tumors presenting with separate invasive lesions. MFBC was not differentiated from MC disease by distance of separation or according to lobar anatomy. Based on the analysis of the genetic mutations in homogenous phenotypic ductal MFBC lesions in terms of grade, estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2), the researchers were able to identify three ‘genomic’ groups. Thirty-one percent of tumors were referred to as the ‘homogeneous’ group where all MF lesions carried the same mutations. The second or ‘intermediate group’, comprising 36% of patients, had both common and private mutations. The third group of patients had no single mutation in common among all samples from the investigated lesions. This last group was categorized as ‘heterogeneous’. The only association of significance between inter-lesion heterogeneity and clinico-pathological characteristics was that of inter-lesion distance. It was noted that the lesions from patients of the heterogeneous group were further apart from each other than those from patients of the homogeneous group. This observation supports the concept of the sick lobe theory, where it is postulated that a susceptible progenitor cell develops during embryology and retains the mutation, which passes on through mitosis to its cell lineage during mammary gland formation at puberty. These aberrant cells are dispersed along the ductal tree and within the TDLUs. The finding of genomic similarity for lesions in close proximity, would therefore be consistent with this hypothesis. The ‘homogenous’ group identified by Desmedt et al. would correspond to true MFBC, where lesions arise from elements within a single lobe. Extrapolating this concept to MC disease, different anomalous progenitor cell lines mature into separate lobes, resulting in heterogeneous mutations. This is consistent with the finding of greater inter-lesion separation. Furthermore, the proportion of patients with lesions sharing at least a fraction of identified mutations by targeted sequencing is concordant with large clinical studies reporting findings relating to MFMCBC. Shared mutations were noted in 67% of patients by Desmedt et al., while Ataseven et al. and Wolters et al. reported MF lesions in 58% & 75.1% of patients with MIBCs, respectively (22,23).
In their report, Desmedt and colleagues recommend that, due to the observed genomic heterogeneity in morphologically homogenous malignant foci, each lesion from patients with MFBC should be individually evaluated, in particular when the lesions are relatively distant from each other. However, no exact inter-lesion dimension was suggested. This lack of a distinct inter-lesion distance is comparable to the absence of a universally accepted clinical definition demarcating MF form MC disease, with MC disease being defined as lesions separated by distances anywhere between 2 and 5 cm (24). In the light of emerging molecular information, the boundaries defining multifocality or multicentricity may not be anatomical, pathological or related to inter-lesion distance, but may be genomic. If so, extent of the ‘sick lobe’ might only be discovered through gene-expression studies identifying the existence of private mutations. The interaction of such individual genetic mutations with the stromal microenvironment could have implications for margin status (25). Recognition of certain tumor mutations, in conjunction with stromal characteristics, could be a factor determining individualized phenotypic ‘clear’ margin in time to come. The ideal physical dimension of a negative margin has been postulated by Leyba et al. to be variable according to the interaction between tumor and tissue biology (25). This could provide insights into margin status and required resection volume and is therefore logically the subject of future research on biomarkers for precision surgical therapy.
Treatment selection
Neoadjuvant chemotherapy
First generation prognostic indicators for breast cancer include tumor size, histologic grade and lymph node status, which continue to play a significant role in decision making for adjuvant treatment. More contemporary methods of classification define molecular phenotypes (luminal A, luminal B, HER2 enriched and triple negative) also influence the use of medical treatment, chiefly whether or not to administer chemotherapy. Neoadjuvant chemotherapy was first mooted in 1985 (26), and its present position as a validated means of preoperative downstaging lays testament to the extensive work on it over the last few decades. Patients with tumors too large for breast conserving surgery at the time presentation may now undergo primary medical treatment for tumor size reduction and potential BCT eligibility (27).
A pragmatic approach to personalized therapy would be to apply subtype appropriate systemic therapy for women presenting with stage II & III breast cancer. Extent of surgery, either mastectomy or BCT, following primary medical treatment, would be directed by tumor response. Subsequent medical treatment may be intensified or de-escalated according to the degree of residual tumor burden. When applied to MFMCBC, this approach has prognostic value (22). Ataseven et al. found no significant difference in local recurrence-free survival in women with complete pathologic response regardless of type of disease (unifocal or MFMCBC) and surgical therapy (22). However, for those without a pathologic complete response, women with MC had a poorer local recurrence-free survival. Nevertheless, combined data from the GeparTrio, GeparQuattro and GeparQuinto studies suggests that BCT is feasible for appropriately selected patients with clinical MFMCBC who undergo neoadjuvant chemotherapy (22).
Recurrence scores using multi-gene assays appear promising in predicting tumor response to primary medical therapy (28). In a study by Bear et al., of patients initially not suitable for BCT, more than 57% underwent successful BCT. Although there was a trend towards higher BCT rates for patients with hormone receptor positive and low recurrence scores, this was not found to be statistically significant. These results provide opportunities for future evaluation of multigene assays in predicting response of MFMCBC to preoperative medical therapy and appropriate case selection for BCT (22,28), offering potential for genomics also to guide individualized de-escalation of surgical therapy.
Radiotherapy
Since the NSABP B-06 study demonstrated significantly reduced local recurrence when WBRT was administered, it was routinely recommended as adjuvant treatment for BCT. In a large study comprising multiple tumor types, including breast cancer, Scott et al. surmised that a genomic-adjusted radiation dose (GARD) clinical model could allow individualization of radiotherapy dose (29). Current consensus recommends the administration of WBRT for MFMCBC (30). With the present improved understanding of genetic mutations in MFMCBC, it is reasonable to anticipate biomarker-guided precision adjuvant radiotherapy when more research data is available.
Extent of surgery
There is adequate contemporary evidence reporting acceptable local control and survival rates with BCT for MFMCBC to support a consensus statement approving the use of BCT for MFMCBC provided clear margins are obtained for all clinically evident tumor foci and WBRT is administered (30). Once these imperative oncologic principles are fulfilled, a secondary requisite is the attainment of a reasonable cosmetic outcome. To date, selection criteria for BCT in patients with MFMCBC are based primarily on clinico-pathologic characteristics. In the foreseeable future, patient selection on a more fundamental level relating to tumor biology and genomics is a tenable reality.
Historically, adequacy of margin was determined by a concentric rim of phenotypically normal tissue, with current guidelines requiring ‘no ink on tumor’ (31). Based on patho-anatomy, it may be logical to resect tumor(s) together with the diseased lobe(s). Since modern imaging technology does not enable the preoperative identification of the ‘sick lobe(s)’, the closest estimate would be the surgical extirpation of a segment, in the case of MF disease, or multiple segments, in the case of MC tumors (32). In larger contemporary studies, BCT rates for MF disease are reported to be between 47.6% and 58.6% while those for MC lesions are between 13% and 30% (22,23). Applying a lobar surgical approach in a small cohort of patients using a segment classification for MFMCBC, BCT rates of 84.6% and 86.7% for MF and MC cancers respectively can be achieved (33). In this small cohort of patients reported by Tan et al., local control and overall survival outcomes for women who had undergone BCT were comparable to other larger studies (22,23,33). Moreover, classifying MFMCBC based on quadrants instead of segments may not have a significant impact on the high rate of successful BCT (34).
Several advantages of BCT for unifocal disease have been identified. Multiple contemporary retrospective, population-based studies have reported data to suggest that women who had undergone BCT have superior outcomes in terms of breast cancer-specific survival and local control when compared to mastectomy (35-42). In addition, complication rates for BCT are reported to be lower than those for mastectomy with or without reconstruction (43,44). Whether treatment de-escalation in the form of increasing BCT for MFMCBC will confer similar benefits to women is a subject for further research. Currently a phase II trial comparing BCT and mastectomy for MIBCs is underway (45). In this study, inclusion and selection criteria are based on clinic-pathologic rather than molecular characteristics. There is therefore, opportunities for research into the use of genomics to inform appropriate intensification and de-escalation of surgical treatment for MFMCBC as these are unavailable at present. It is conceivable that refinement of selection criteria through integration of genomic capabilities with conventional subtyping will allow individualized surgical therapy in terms of extent and margin status.
Conclusions
It is recognized that surgery for breast disease has evolved significantly in the decades that followed the first RCTs comparing BCT and mastectomy, in a large part due to concomitant scientific progress in the other disciplines involved in breast oncologic care. Pathology plays a dominant role, and together with breast screening, has enabled less extensive and mutilating surgery without sacrificing survival outcomes. Modern medicine has moved into a new era with genomic technology. The use of molecular biomarkers for treatment selection is already a clinical reality in the field of medical oncology (46,47). While its influence in surgery has been acknowledged for a significant period of time (48-50), apart from BRCA mutations to guide prophylactic surgery, there has not been a breakthrough in its use to the degree where surgical decision making for breast cancer is routinely informed by tumor biomarkers (51).
It has been several decades since BCT was established as appropriate surgical treatment for early breast cancer. Despite this, there is a perplexing trend of increasing mastectomy and contralateral prophylactic mastectomy rates (52,53), the latter possibly constituting overtreatment in BCT-eligible patients (54). Improved understanding of tumor biology should enable a reduction in such overtreatment. Traditionally, MFMCBC was a contraindication to BCT. However, a growing body of evidence demonstrates that BCT is appropriate in the setting of MFMCBC provided there is a process of careful patient selection. Current selection criteria are based on traditional clinic-pathologic characteristics. Genomic technology holds great promise not only for prognostication but also for prediction of surgical outcomes, and may be applied in the clinical setting in several ways. Firstly, molecular profiling of the tumor can inform risk of local or regional recurrence, prompting appropriate intensification or de-escalation of surgery. Secondly, the identification of specific genetic mutations at the margins of phenotypically normal tissue may indicate need for wider margins. Alternatively, the relative proportions of cells with or without mutations may indicate adequacy of resection volume. Thirdly, biomarkers might provide insights into the configuration of the ‘sick lobe’ to guide resection patterns. As a corollary, the absence of biomarkers would indicate the adequacy of less extensive procedures. Hence, personalized risk stratified extent of surgery and margin width is a distinct possibility with the appropriate integration of genomics in clinical practice, for both unifocal and MFMCBC.
In contrast to overtreatment rendered in the past to compensate for rudimentary technology, there is scope to exploit the capabilities offered in the present genomic era to optimize and individualize surgical therapy. This review has identified contemporary disparity in the use of genomics for medical and surgical intervention for breast cancer. Selection criteria for tailored medical therapy using multi-gene assays is now standard of care for various molecular subtypes of breast cancer. In comparison, there is a paucity of evidence with respect to biomarkers and surgical decision making. It is evident, therefore, that there are tremendous opportunities for research into the use of biomolecular markers to predict survival and local, regional and distant recurrence risk to serve as reliable guides to local, regional and systemic therapy. Integration of clinical data, histologic information, surgical techniques and molecular profiling offer potential for individualized, precision surgery in MFMCBC in the genomic era (55).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2020.02.01). MPT serves as an unpaid editorial board member of Annals of Breast Surgery from Aug 2019 to Jul 2021.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bloodgood JC. Chronic cystic mastitis of the diffuse, non-encapsulated, cystic adenomatous type. Ann Surg 1929;90:886-903. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Rosen PP, Gracchia AA, Urban JA, et al. “Residual” mammary carcinoma following simulated partial mastectomy. Cancer 1975;35:739-47. [Crossref] [PubMed]
- Lagios MD. Multicentricity of breast carcinoma demonstrated by routine correlated serial subgross and radiographic examination. Cancer 1977;40:1726-34. [Crossref] [PubMed]
- Holland R, Veling SH, Mravunac M, et al. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer 1985;56:979-90. [Crossref] [PubMed]
- Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312:665-73. [Crossref] [PubMed]
- Engelsman E, Persijn JP, Korsten CB, et al. Oestrogen receptor in human breast cancer tissue and response to endocrine therapy. Br Med J 1973;2:750-2. [Crossref] [PubMed]
- Todd JH, Dowle C, Williams MR, et al. Confirmation of a prognostic index in primary breast cancer. Br J Cancer 1987;56:489-92. [Crossref] [PubMed]
- Bonadonna G, Valagussa P, Moliterni A, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 1995;332:901-6. [Crossref] [PubMed]
- Watson JD, Crick FH. Molecular structure of nucleic acids. Nature 1953;171:737-8. [Crossref] [PubMed]
- Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature 2001;409:860-921. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- NIH consensus conference. Treatment of early-stage breast cancer. JAMA 1991;265:391-5. [Crossref] [PubMed]
- Kurtz JM, Jacquemier J, Amalric R, et al. Breast-conserving therapy for macroscopically multiple cancers. Ann Surg 1990;212:38-44. [Crossref] [PubMed]
- Wilson LD, Beinfield M, McKhamn CF, et al. Conservative surgery and radiation in the treatment of synchronous ipsilateral breast cancers. Cancer 1993;72:137-42. [Crossref] [PubMed]
- American College of Radiology. Practice guideline for the breast conservation therapy in the management of invasive breast carcinoma. J Am Coll Surg 2007;205:362-76. [Crossref] [PubMed]
- Going JJ, Mohun TJ. Human breast duct anatomy, the ‘sick lobe’ hypothesis and intraductal approaches to breast cancer. Breast Cancer Res Treat 2006;97:285-91. [Crossref] [PubMed]
- Tot T. The limited prognostic value of measuring and grading small invasive breast carcinomas: the whole sick lobe versus the details within it. Med Sci Monit 2006;12:RA170-5. [PubMed]
- Tot T. The theory of the sick breast lobe and the possible consequences. Int J Surg Pathol 2007;15:369-75. [Crossref] [PubMed]
- Desmedt C, Fumagalli D, Pietri E, et al. Uncovering the genomic heterogeneity of multifocal breast cancer. J Pathol 2015;236:457-66. [Crossref] [PubMed]
- Ataseven B, Lederer B, Blohmer JU, et al. Impact of multifocal of muliticentric disease on surgery and locoregional. Distant and overall survival of 6.134 breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol 2015;22:1118-27. [Crossref] [PubMed]
- Wolters R, Wockel A, Janni W, et al. Comparing the outcome between multicentric and multifocal breast cancer: what is the impact on survival, and is there a role for guideline-adherent adjuvant therapy? A retrospective multicentre cohort study of 8,935 patients. Breast Cancer Res Treat 2013;142:579-90. [Crossref] [PubMed]
- Kapoor NS, Chung A, Huynh K, et al. Preliminary results: double lumpectomies for multicentric breast carcinoma. Am Surg 2012;78:1345-8. [PubMed]
- Lebya K, Garcia-Smith R, Swaminathan R, et al. Towards a personalized surgical margin for breast conserving surgery-Implications of field cancerization in local recurrence. J Surg Oncol 2017;115:109-15. [Crossref] [PubMed]
- Ragaz J, Baird R, Rebbeck P, et al. Neoadjuvant (preoperative) chemotherapy for breast cancer. Cancer 1985;56:719-24. [Crossref] [PubMed]
- Criscitiello C, Curigliano G, Burstein HJ, et al. Breast conservation following neoadjuvant therapy for breast cancer in the modern era: are we losing the opportunity? Eur J Surg Oncol 2016;42:1780-6. [Crossref] [PubMed]
- Bear HD, Wan W, Robidoux A, et al. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: a multicenter trial. J Surg Oncol 2017;115:917-23. [Crossref] [PubMed]
- Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol 2017;18:202-11. [Crossref] [PubMed]
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the primary therapy of early breast cancer 2015. Ann Oncol 2015;26:1533-46. [Crossref] [PubMed]
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol 2014;21:704-16. [Crossref] [PubMed]
- Tan MP. A novel segment classification for multifocal and multicentric breast cancer to facilitate breast-conservation treatment. Breast J 2015;21:410-7. [Crossref] [PubMed]
- Tan MP, Sitoh NY, Sitoh YY. Optimising breast conservation treatment for multifocal and multicentric breast cancer: a worthwhile endeavour? World J Surg 2016;40:315-22. [Crossref] [PubMed]
- Tan MP, Sitoh NY, Sitoh YY. Optimising breast conservation treatment for multifocal and multicentric breast cancer: a worthwhile endeavour? World J Surg 2017;41:346-7. reply. [Crossref] [PubMed]
- Hwang ES, Lichtensztajn DY, Gomez SL, et al. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 2013;119:1402-11. [Crossref] [PubMed]
- Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg 2014;149:267-74. [Crossref] [PubMed]
- van Hezewijk M, Bastiaannet E, Putter H, et al. Effect of local therapy on locoregional recurrence in postmenopausal women with breast cancer in the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial. Radiotherapy and Oncology 2013;108:190-6. [Crossref] [PubMed]
- Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol 2011;29:2852-8. [Crossref] [PubMed]
- Hofvind S, Holen A, Aas T, et al. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumour characteristics. Eur J Surg Oncol 2015;41:1417-22. [Crossref] [PubMed]
- van der Heiden-van der Loo M, Siesling S, Wouters MW, et al. The value of ipsilateral breast tumor recurrence as a quality indicator: hospital variation in the Netherlands. Ann Surg Oncol. 2015;22:S522-8. [Crossref] [PubMed]
- Hartmann-Johnsen OJ, Karesen R, Schlichting E, et al. Survival is better after breast conserving therapy than mastectomy for early stage breast cancer: a registry-based follow-up study of Norwegian women primary operated between 1998-2008. Ann Surg Oncol 2015;22:3836-45. [Crossref] [PubMed]
- van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 2016;17:1158-70. [Crossref] [PubMed]
- Chatterjee A, Pyfer B, Czerniecki B, et al. Early postoperative outcomes in lumpectomy versus simple mastectomy. J Surg Res 2015;198:143-8. [Crossref] [PubMed]
- Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer. Ann Surg 2016;263:219-27. [Crossref] [PubMed]
- Rosenkranz KM, Ballman K, McCall L, et al. The feasibility of breast-conserving surgery for multiple ipsilateral breast cancer: an initial report from ACOSOG Z11102 (Alliance) trial. Ann Surg Oncol 2018;25:2858-66. [Crossref] [PubMed]
- Paik S. Development and clinical utility of a 21-gene recurrence score prognostic assay in paitents with early breast cancer treated with tamoxifen. The Oncologist 2007;12:631-5. [Crossref] [PubMed]
- Lumachi F, Chiara GB, Foltran L, et al. Proteomics as a guide for personalised adjuvant chemotherapy in patients with early breast cancer. Cancer Genomics Proteomics 2015;12:385-90. [PubMed]
- Brunicardi FC. Molecular surgery and biology. Am J Surg 2000;180:397-401. [Crossref] [PubMed]
- Boughey JC, Margenthaler JA. How genomics, research, ethics and advances translate into improved care for breast surgery patients. Ann Surg Oncol 2013;20:3153-7. [Crossref] [PubMed]
- Beggs AD, Dilworth MP. Surgery in the era of the 'omics revolution. Br J Surg 2015;102:e29-40. [Crossref] [PubMed]
- Ziogas D, Roukos DH. Genetics and personal genomics for personalised breast cancer surgery: progress and challenges in research and clinical practice. Ann Surg Oncol 2009;16:1771-82. [Crossref] [PubMed]
- Dragun AE, Huang B, Tucker TC, et al. Increasing mastectomy rates among all age groups for early stage breast cancer: a 10-year study of surgical choice. Breast J 2012;18:318-25. [Crossref] [PubMed]
- Soran A, Polat AK, Johnson R, et al. Increasing trend of contralateral prophylactic mastectomy: what are the factors behind this phenomenon? Surgeon 2014;12:316-22. [Crossref] [PubMed]
- Donovan CA, Bao J, Gangi A, et al. Bilateral mastectomy as overtreatment for breast cancer in women age forty years and younger with unilateral operable invasive breast cancer. Ann Surg Oncol 2017;24:2168-73. [Crossref] [PubMed]
- Tan MP, Tot T. The sick lobe hypothesis, field cancerisation and the new era of precision breast surgery. Gland Surg 2018;7:611-8. [Crossref] [PubMed]
Cite this article as: Tan MP. Precision surgery for multifocal multicentric breast cancer in the genomic era. Ann Breast Surg 2020;4:6.

