
Nipple adenoma: a review of the literature
Introduction
Nipple adenomas (NA) were first described by Handley and Thackray in 1962 and are benign and rare epithelial proliferations that arise from the lactiferous ducts of the nipple (1). They are also known as ‘erosive adenomas’, ‘subareolar adenomas’, ‘syringomatous adenomas’ or may be diagnosed as the conditions ‘erosive adenomatosis’ or ‘florid papillomatosis’ of the nipple.
They most commonly present as a smooth nodule on the nipple however nipple discharge, hyperkeratosis, deformity, ulceration and erosion have also all been described. A clinical diagnosis can be challenging and histological confirmation is essential to exclude other differentials such as Paget’s disease of the nipple or carcinoma (2-4).
The lesion most commonly presents in females in their fourth decade of life but has been reported in men, adolescents and infants (5-7). NA are relatively small in size, ranging from 0.5–3.5 cm (8). They are benign lesions that can locally infiltrate smooth muscle and nerves within the nipple stroma, but do not metastasise. Much of the literature regarding NA consists solely of case-reports and case-series (Table 1).
Table 1
| Literatures | Myoepithelial marker | Epithelial marker | Histological type | Duct distortion | Treatment | Follow up |
|---|---|---|---|---|---|---|
| Di Bonito et al. | p63, α-smooth muscle actin, caldesmon, calponin, CD10, CK5/6 | CK 8 + CK18 | Epithelial hyperplasia | Fibrosis | Excision of NAC | – |
| Ishikawa et al. | Ki-67 | Ki-67 | – | Tear drop, comma-shaped | Enucleation | Ongoing |
| Spohn et al. | p63, smooth muscle myosin heavy chain | CK5 | Adenosis | Sclerosis | Central excision | Ongoing (31 months) |
| Barco et al. | p63 | – | Epithelial hyperplasia | – | MOHS | Ongoing (annual) |
| Tuveri et al. | Calponin, muscle actin | CK7, MUC1 | Epithelial hyperplasia | – | Excision of NAC | 4 years |
| Odashiro et al. | 34 β E12 | – | – | Comma shaped | – | 1 year |
| Yosepovich et al. | Smooth muscle actin | – | – | Comma shaped, tadpole shaped | Excisional biopsy + re-excision | 2 years |
| Ku et al. | Smooth muscle actin | Cytokeratin (Cam 5.2) | Mixed | Tear drop + comma shaped | Wide excision | 5 years |
NAC, nipple-areolar complex; NA, nipple adenoma.
Clinical presentation
NA are uncommon and most breast units can expect to see approximately one adenoma per year (3,9,10). NA tend to occur on one side only, however, a case of bilateral disease has been reported (10,11).
Most NA present with a palpable lesion with some degree of nipple distortion with or without crusting, erythema, thickening, itching, inflammation, ulceration, discharge, and pain (12). In one case-series, 83% of patients were symptomatic from the NA with nipple discharge being the most prevalent symptom (58.3%) and is commonly serous or sanguineous (9,13).
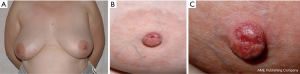
Duration of symptoms is highly variable and can extend from months to several years. NA may erode through the nipple epidermis which can give an appearance identical to Paget’s disease of the nipple, ductal carcinoma in situ or invasive ductal carcinoma (12,13) (Figure 1).
Histopathology
The World Health Organisation (2012 version) define NA as “a proliferation of small tubules lined by epithelial and myoepithelial cells, with or without proliferation of the epithelial component, around the collecting ducts of the nipple”.
Proliferation of the lactiferous ducts of the nipple is the most characteristic finding in NA. Ducts are characteristically lined by double layers of myoepithelial and epithelial cells and are often described as being tear-drop or comma shaped due to duct distortion as result of cellular growth, fibrosis and sclerosis (2,3,14).
The presence of a dual cell layer is the main histological characteristic that helps distinguish NA from invasive or in situ disease.
Three main histological phenotypes of NA are recognised and all three types can exist within the same NA as a mixed form. Epithelial hyperplasia type: significant duct hyperplasia is present, often in recognised patterns such as tubular, solid, papillary and pseudo-cribiform. Necrosis may be present (10,15).
Adenosis type: the NA is present within the dermis and has clear margins. Glandular ducts contain two types of cell, epithelium and myoepithelium. Pseudo-infiltrating or pseudo-invasive type: infiltrative pattern with distorted ducts and squamous cysts with no epithelial hyperplasia (4,16).
Other histological features often present in NA include apocrine metaplasia, adenosis, papillary hyperplasia, squamous metaplasia, acanthosis, eosinophilic cytoplasm, low mitotic figure count and keratin cysts (3,13). Like many benign proliferative lesions of breast parenchyma, PIK3CA and RAS mutations are frequently identified in NA, in one series over 50% of cases [PIK3CA (17)].
Fine needle aspiration cytology (FNAC) or tissue biopsies are accurate and recognised techniques for tissue diagnosis of NA. FNAC samples often reveal large number of cells with populations of epithelial and myoepithelial cells, containing fine chromatin and uniform nucleoli (5).
Immunohistochemical analysis should be performed on all specimens for specific markers associated with myoepithelium. Many cell markers have been described in the literature to identify NA: α-smooth muscle actin, smooth muscle myosin heavy chain, calpronin, CD10, p63 and CK5/6. The presence of two markers is sufficient for diagnosis, with p63 and CK5/6 being the most common and specific (2-4,13,18).
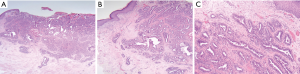
NA can be mis-diagnosed as ductal carcinoma due to the presence of sclerosis, necrosis and a pseudo-invasive appearance on histology due to fibrosis (18). A lack of myoepithelial cells within the specimen and/or positive staining for malignant cell markers e.g., c-erbB-2, p53, Ki-67, HER2 and hormone receptors (ER/PR) can help distinguish NA from malignant disease (4,8,19) (Figure 2).
Imaging
All mainstream breast imaging modalities such as ultrasound (USS), mammography and magnetic resonance imaging (MRI) have and can be used to help diagnose and characterise NA. USS remains the most clinically useful imaging modality as it can help define soft tissue nodularity, assess internal vascularity and also allows image-guided FNA. The lesion is often well defined and hypoechoic on USS and may demonstrate posterior echo enhancement (2,4,19).
The use of MRI to aid diagnosis of NA has been reported in the literature in several case-reports. The MRI enhancement-curve for NA is similar to malignancy in that NA tend to demonstrate rapid uptake with a peak of 60–90 secs followed by washout and for this reason, may not be helpful in diagnosis (20,21). Within the literature, the majority of cases were imaged with USS and mammography only (12,14,18,22,23).
Mammography gives heterogeneous results that can range from normal or unspecific changes e.g., benign calcification to a dense lesion with well-defined or irregular margins (22). Due to the heterogenicity of findings that can be demonstrated, no one imaging modality should be used in isolation for the diagnosis of NA.
Treatment
Surgical excision is the only treatment for NA and many surgical techniques have been described in the literature (Table 1). NA often re-occur if incompletely excised but reoccurrence is rare following complete excision (5,8,17,18).
Some authors advocate complete excision of the nipple to ensure clear margins, whilst others perform a more radical central breast excision (8,13,24). In these cases, the patients who are often young, will require further surgery to reconstruct the nipple and report low satisfaction and poor cosmesis.
Nipple preserving techniques (wedge resection, enucleation, MOHS micrographic surgery, cryosurgery) have been shown to be just as effective in terms or re-occurrence but are dependent on the size of the NA and its position within the nipple (2,4,10,11,16). As NA often occurs within the dermis, complete resection of the nipple may be required to avoid reoccurrence. Recurrence rates have been reported to be as high as 25–55% following incomplete excision (12) (Table 1).
Association with breast cancer
Despite being a benign lesion, there is a relationship between NA and breast cancer. In a series of 51 patients with florid papillomatosis of the nipple, 17% of patients had active breast cancer and 12% of patients having a mastectomy for breast cancer were found to have NA (25).
In a case-series of five patients with breast cancer, NA was present and in three of the cases, investigation of symptoms from the NA led to diagnosis of clinically-occult invasive disease (26). Finally, in a case-series of 12 patients with NA, at 3 years follow up post excision, three patients (25%) had been diagnosed with breast cancer prior to or post diagnosis of a nipple adenoma (10).
Due to the high prevalence of breast cancer and the low prevalence of NA, a causal link has not been demonstrated between the two. Indeed, malignant transformation has been documented only once in the literature and remains a very rare occurrence (27). The presence of a NA should heighten the suspicion of a co-existent breast cancer and all patients should be appropriately screened with triple-assessment prior to definitive treatment for the NA.
Follow up
No formal consensus exists for how long patients should be monitored following adenoma excision and remains at the discretion of the operating surgeon and the histological evaluation of margins. If negative margins are achieved, re-occurrence is rare.
In their study of patients with negative margins, Suster et al. found no evidence of recurrence in patients at 6 years follow up (28). In many of the case series and reports in the literature, authors report that many patients post adenoma excision remain under active monitoring. Local recurrence rates of nipple adenoma following excision with positive margins are high with time to re-occurrence ranging from 1.5–4 years.
The authors suggest a follow-up policy on a patient by patient basis centred around a discussion within the whole breast multi-disciplinary team. Patients with negative margins, no re-occurrence at one year and low risk for breast malignancy could be discharged to patient-initiated follow up (PIFU) for example. Patients with NA and a family or personal history of breast disease that raises the risk of breast cancer (e.g., indeterminate B3 lesions, moderate to high risk family’s) a period of radiological and clinical follow up seems appropriate. Patients with a high risk of NA re-occurrence (positive margins and refusal for further excision, previous NA re-occurrence) again my benefit from a PIFU policy. In all patients, education regarding regular breast self-examination, attendance at screening and the importance of reporting new or changing breast symptoms remains essential (8,22,26,29).
Conclusions
NA are rare benign lesions that can behave in a locally invasive manner. They do not metastasise. Accurate histological and immunohistochemical analysis is essential to help delineate pseudo-invasion, a common finding in NA, from invasive carcinoma. Treatment is with surgical excision and fully excised lesions rarely re-occur. The nipple can be spared safely in selected patients. We recommend a follow up policy on a case by case basis with involvement of the MDT for these rare breast lesions.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.10.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Handley RS, Thackray AC. Adenoma of Nipple. Br J Cancer 1962;16:187-94. [Crossref] [PubMed]
- Fujii T, Yajima R, Morita H, et al. Adenoma of the nipple projecting out of the nipple: curative resection without excision of the nipple. World J Surg Oncol 2014;12:91. [Crossref] [PubMed]
- DI Bonito M. Adenoma of the nipple: a clinicopathological report of 13 cases. Oncol Lett 2014;7:1839-42. [Crossref] [PubMed]
- Li M, Du J, Wang LJ, et al. A case of Nipple Adenoma Detected by Sonography. Chin Med J 2016;129:2386-7. [Crossref] [PubMed]
- Gupta RK, Dowle CS, Naran S, et al. Fine-Needle Aspiration Cytodiagnosis of Nipple Adenoma (Papillomatosis) in a Man and Woman. Diagn Cytopathol 2004;31:432-33. [Crossref] [PubMed]
- Tao W, Kai F, Hua L. Nipple adenoma in an adolescent. Pediatr Dermatol 2010;27:399-401. [Crossref] [PubMed]
- Clune JE, Kozakewich HP, VanBeek CA, et al. Nipple adenoma in infancy. J Pediatr Surg 2009;44:2219-22. [Crossref] [PubMed]
- Carter E, Dyess D. Infiltrating Syringomatous Adenoma of the Nipple: a Case Report and 20-Year Retrospective Review. Breast J 2004;10:443-7. [Crossref] [PubMed]
- Dy B, Tortorelli C, Shah S, et al. Nipple adenoma. J Surg Radio 2011;2:86-91.
- Wang C, Wang X, Ma R. Diagnosis and surgical treatment of nipple adenoma. ANZ J Surg 2015;444-7. [PubMed]
- Kuflik EG. Erosive adenomatosis of the nipple treated with cryosurgery. J Am Acad Dermatol 1998;38:270-1. [Crossref] [PubMed]
- Ku J, Bennett R, Chong K, et al. Syringomatous adenoma of the nipple. Breast 2004;13:412-5. [Crossref] [PubMed]
- Spohn GP, Trotter SC, Tozbikian G, et al. Nipple adenoma in a female patient presenting with persistent erythema of the right nipple: case report, review of the literature, clinical implications, and relevancy to health care providers who evaluate and treat patients with dermatologic conditions of the breast skin. BMC Dermatol 2016;16:4. [Crossref] [PubMed]
- Oo KZ, Xiao P. Infiltrating Syringomatous Adenoma of the Nipple. Arch Pathol Lab Med 2009;133:1487-9. [PubMed]
- Ozaki S, Mizukami Y, Kawahara E. Cytologic Features of Nipple Adenoma: a Report of Four Cases of Adenoma of the Nipple. Diagn Cytopathol 2015;43:664-8. [Crossref] [PubMed]
- Barco I, Vidal M, Fraile M, et al. MOHS micrographic surgery for treating erosive adenoma of the nipple: a case report and review of the literature. Int J Dermatol 2017;56:1451-4. [Crossref] [PubMed]
- Liau JY, Lee YH, Tsai JH, et al. Frequent PIK3CA activating mutations in nipple adenomas. Histopathology 2017;70:195-202. [Crossref] [PubMed]
- Tuveri M, Calo P, Mocci C, et al. Florid papillomatosis of the male nipple. Am J Surg 2010;200:e39-e40. [Crossref] [PubMed]
- Yosepovich A, Perelman M, Ayalon S, et al. Syringomatous adenoma of the nipple: a case report. Pathol Res Pract 2005;201:405-7. [Crossref] [PubMed]
- Matsubayashi RN, Adachi A, Yasumori K, et al. Adenoma of the nipple: correlation of magnetic resonance imaging findings with histologic features. J Comput Assist Tomogr 2006;30:148-50. [Crossref] [PubMed]
- Buadu AA, Buadu LD, Murakami J, et al. Enhancement of the nipple-areolar complex on contrast-enhanced MR imaging of the breast. Breast Cancer 1998;5:285-9. [Crossref] [PubMed]
- Odashiro M, Lima MG, Miiji LN, et al. Infiltrating Syringomatous Adenoma of the Nipple. Breast J 2009;15:414-6. [Crossref] [PubMed]
- AlSharif S, Tremblay F, Omeroglu A, et al. Infiltrating syringomatous adenoma of the nipple: Sonographic and mammographic features with pathologic correlation. J Clin Ultrasound 2014;42:427-9. [Crossref] [PubMed]
- Ishikawa S, Sako H, Masuda K, et al. Syringomatous adenoma of the nipple: a case report. J Med Case Rep 2015;9:256-9. [Crossref] [PubMed]
- Rosen PP, Caicco JA. Florid papillomatosis of the nipple. A study of 51 patients, including nine with mammary carcinoma. Am J Surg Pathol 1986;10:87-101. [Crossref] [PubMed]
- Jones MW, Tavassoli FA. Coexistence of nipple duct adenoma and breast carcinoma: a clinicopathologic study of five cases and review of the literature. Mod Pathol 1995;8:633-6. [PubMed]
- Gudjónsdóttir A, Hägerstrand I, Ostberg G. Adenoma of the nipple with carcinomatous development. Acta Pathol Microbiol Scand A 1971;79:676-80. [PubMed]
- Suster S, Moran CA, Hurt MA. Syringomatous squamous tumors of the breast. Cancer 1991;67:2350-5. [Crossref] [PubMed]
- Jones MW, Norris HJ, Snyder RC. Infiltrating syringomatous adenoma of the nipple. A clinical and pathological study of 11 cases. Am J Surg Pathol 1989;13:197-201. [Crossref] [PubMed]
Cite this article as: Tatterton MR, Fiddes R. Nipple adenoma: a review of the literature. Ann Breast Surg 2019;3:29.

