
Necrotizing soft tissue infection of the breast: bilateral presentation in a male patient
Introduction
Necrotizing soft tissue infections (NSTIs) are rare but highly lethal infections that involve necrotizing changes in any of the layers of the soft tissue compartments, including dermis, subcutaneous tissue, fascia, or muscle (1). The condition is associated with significant mortality, and delayed diagnosis is inversely related to patient survival (2). NSTIs can present in any part of the body but are exceptionally rare in the breasts, with only 36 case reports of NSTIs of the breast in the literature (3-16). These infections represent challenging diagnoses, since they often present like common breast pathologies, such as mastitis or abscess, but then continue to progress even after broad-spectrum antibiotic therapy (17). Of the reported cases, all occurred in female patients, and only five affected the breasts bilaterally (7,10,11,13,15). Here, we present the first case of bilateral breast NSTI in a male patient.
Case presentation
A 65-year-old male presented to the emergency department with 24 hours of altered mental status. He had one episode of diarrhea 24 hours prior to presentation, but no other relevant clinical history. The patient’s past medical history was significant for hypertension and stroke. Of note, he did have a history of staphylococcal mastitis of the left breast 30 years prior. Furthermore, he reported history of draining wounds of the bilateral breasts 1.5 years prior after scratching both breasts on a fence. After the wounds healed, he developed bilateral, subareolar calcifications and induration, which prompted additional evaluation. He underwent a mammogram, which was normal, as well as bilateral punch biopsies of the breast, which showed ulceration and dermal sclerosis, but no evidence of malignancy.
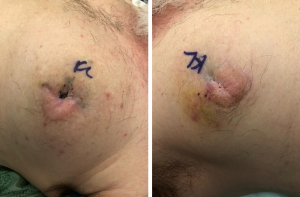
Subjectively, the patient denied breast pain. On examination, his breasts were non-erythematous (Figure 1) and nontender to palpation. His vital signs were within normal limits. His laboratory values were notable for a white blood cell count of 13.8 K/µL, hemoglobin of 15.4 g/dL, sodium of 137 mmol/L, creatinine of 0.9 mg/dL, glucose of 140 mg/dL, and C-reactive protein of 1.28 mg/dL. The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score, which suggests a diagnosis of NSTI when greater than or equal to 6 (18), was 0. Because the cause of altered mental status was unclear, the patient underwent a diagnostic chest X-ray, which demonstrated subcutaneous gas. A subsequent CT scan of the chest demonstrated a significant amount of gas underneath the breasts bilaterally with some dermal thickening at the level of the nipple-areola complex (Figure 2). Gas extended to the pectoralis major muscle bilaterally. There was no pneumothorax, mediastinal lymphedema, or evidence of rib fractures to suggest the gas had originated in the chest cavity.
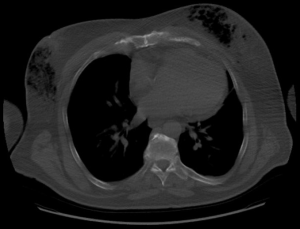
This clinical picture of altered mental status and imaging findings created high suspicion for NSTI of the breast. He was emergently taken to the operating room for debridement via bilateral mastectomy. Antibiotics, which were started shortly after presentation, were broadened to include clindamycin. Intraoperatively, subcutaneous liquefactive necrosis was noted but the fascia and pectoralis muscle showed no signs of infection bilaterally. Culture of the breast tissue grew Enterococcus faecalis, Staphylococcus epidermis, and coagulase negative Staphylococcus. The final pathology report confirmed subcutaneous necrosis with surrounding dermal sclerosis.
The patient’s mental status improved significantly postoperatively, suggesting that his behavioral changes were secondary to toxic metabolite encephalopathy in the setting of sepsis related to the NSTI. Four weeks postoperatively, his incisions were well-healed without signs of persistent infection. A colonoscopy, which was recommended due to concern for possible colonic translocation in the setting of E. faecalis on cultures, was normal.
Discussion
NSTIs are rare, with an incidence of 0.04 cases per 1,000 person-years in the United States (1). NSTIs of the breast are even more uncommon with less than 40 cases reported. Typical clinical characteristics of NSTI include swelling, erythema, pain, and tachycardia. Upon progression, tense edema, pain disproportionate to appearance, ecchymosis, blisters/bullae, crepitus and/or subcutaneous gas, and shock may also be observed. Unfortunately, these findings carry fairly low sensitivity and are only evident in 10–40% of NSTI cases (1). In the breasts, the early findings of NSTI can appear similar to a developing mastitis, abscess, or carcinoma. Reported cases of breast NSTI were ultimately diagnosed based on the development of gross necrotic changes, imaging findings, or an elevated LRINEC score.
In the breasts, NSTIs have been noted after mastectomy (5,11), other surgery, core needle biopsy (3,5,9-11,13,15,16), abscess drainage (15), or spontaneously (5,8,11,14). Our patient followed this pattern, with his history of bilateral punch biopsies. Only one other case of breast NSTI documented a history of past mastitis like our patient, but in this case, the patient presented with pain, swelling, black discoloration, and visibly necrotic areas (6).
Breast anatomy further complicates expedient diagnosis of NSTI in this area. Due to the greater amount of tissue between the fascia and the skin, cutaneous signs of a necrotizing infection may not be observable until later in the disease process (4). Our patient’s mild gynecomastia may explain the lack of skin changes that might have visibly signaled necrotizing change and underscores the importance of imaging in establishing his diagnosis. Treatment focuses on fluid resuscitation, antibiotic therapy, and surgical debridement of the necrotic tissue until healthy bleeding is visualized in all directions (8). Frequently, mastectomy is the appropriate treatment by the time the diagnosis has been made.
NSTIs of the breast are typically characterized by polymicrobial infections. This pattern holds true for our patient and the other bilateral breast cases, all of which were polymicrobial except for one (14,19). Three reports note enterococcal NSTI of the breast specifically, including two unilateral cases (3,5) and one bilateral case (13). Table 1 summarizes the other pertinent details of the bilateral cases. Explanations for the bilateral nature of the infection included histories of bilateral breast procedures (10,13,15), patient risk factors that promoted systemic infection (7), and a surgical site infection on the inner quadrant of the breast whose location enabled contralateral spread (11). Our patient reported a history of bilateral scratches to the breasts followed by bilateral breast biopsies. Both of these events occurred over a year prior to when he displayed signs of infection. The episode of diarrhea 24 hours before his presentation could have created a transient bacteremia leading to infection. The hematogenous dissemination of bacteria has been shown to cause bilateral breast abscesses, and we hypothesize that a similar process could have precipitated the bilateral NSTI in this case (20).
Table 1
| Author | Year | Sex | Age | Relevant patient characteristics | Culture | Explanation for bilateral infection |
|---|---|---|---|---|---|---|
| Rehman et al. (7) | 2012 | F | 54 | Lupus, steroid therapy, ESRD | Serratia marcescens | Possible break in skin, or hematogenous dissemination facilitated by chronic dialysis and steroid use |
| Yusuf et al. (10) | 2014 | F | 27 | – | Streptococcus pyogenes | History bilateral breast augmentation |
| Angarita et al. (11) | 2014 | F | 43 | ER/PR+, HER2+. Developed surgical site infection after lumpectomy | Gram positive cocci, oxacillin resistant micrococcus, multi-drug sensitive Streptococcus milleri | Surgical site was on inner quadrant of breast, where there is less space between the skin and fascia than the outer quadrant: allowed contralateral spread |
| Pek et al. (13) | 2015 | F | 27 | – | Proteus mirabilis, Enterobacter aerogenes, Enterococcus faecalis, Bacteroides fragilis | History bilateral fat grafting for breast augmentation |
| ALShareef et al. (15) | 2018 | F | 60 | – | “All cultures were negative due to antibiotic use” | History core biopsies for bilateral breast masses |
NSTI, necrotizing soft tissue infection; ESRD, end-stage renal disease.
Conclusions
In conclusion, we report the first case of a bilateral NSTI of the breasts of a male patient. This patient’s presentation was exceptionally distinct not only due to his sex and bilateral infection pattern, but also due to his presentation of altered mental status with subcutaneous emphysema, in the absence of other symptoms. Giving the potential lethality of these infections, the case emphasizes the importance of considering breast NSTI in the differential diagnosis of appropriate patients with an unusual presentation and imaging consistent with a necrotizing process.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.08.04). LGW is a minority stock owner for Elucent Medical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained for the patient for publication of this Case Report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anaya DA, Dellinger EP. Necrotizing Soft-Tissue Infection: Diagnosis and Management. Clin Infect Dis 2007;44:705-10. [Crossref] [PubMed]
- Rouse TM, Malangoni MA, Schulte WJ. Necrotizing fasciitis: a preventable disaster. Surgery 1982;92:765-70. [PubMed]
- Rangaswamy M. Necrotizing fasciitis: a 10-year retrospective study of cases in a single university hospital in Oman. Acta Trop 2001;80:169-75. [Crossref] [PubMed]
- Wong CH, Tan BK. Necrotizing Fasciitis of the Breast. Plast Reconstr Surg 2008;122:151e-2e. [Crossref] [PubMed]
- Aloisio da Costa Vieira R, Zucca Mathes AG, Michelli RA, et al. Necrotizing soft tissue infection of the breast: case report and literature review. Surg Infect (Larchmt) 2012;13:270-5. [Crossref] [PubMed]
- Ablett DJ, Bakker-Dyos J, Rainey JB. Primary necrotizing fasciitis of the breast: a case report and review of the literature. Scott Med J 2012;57:60. [Crossref] [PubMed]
- Rehman T, Moore TA, Seoane L. Serratia marcesens Necrotizing Fasciitis Presenting as Bilateral Breast Necrosis. J Clin Microbiol 2012;50:3406-8. [Crossref] [PubMed]
- Kaczynski J, Dillon M, Hilton J. Breast necrotising fasciitis managed by partial mastectomy. BMJ Case Rep 2012; [Crossref] [PubMed]
- Roque DR, MacLaughlan S, Tejada-Berges T. Necrotizing Infection of the Breast after Core Needle Biopsy. Breast J 2013;19:201-2. [Crossref] [PubMed]
- Yusuf E, Steinrücken J, Nordback S, et al. Necrotizing Fasciitis After Breast Augmentation. Am J Clin Pathol 2014;142:269-72. [Crossref] [PubMed]
- Angarita FA, Acuna SA, Torregrosa L, et al. Bilateral necrotizing fasciitis of the breast following quadrantectomy. Breast Cancer 2014;21:108-14. [Crossref] [PubMed]
- Kohayagawa Y, Ishitobi N, Yamamori Y, et al. Streptococcal toxic shock syndrome from necrotizing soft-tissue infection of the breast caused by a mucoid type strain. J Infect Chemother 2015;21:144-7. [Crossref] [PubMed]
- Pek CH, Lim J, Ng HW, et al. Extensive Necrotizing Fasciitis after Fat Grafting for Bilateral Breast Augmentation: Recommended Approach and Management. Arch Plast Surg 2015;42:365-7. [Crossref] [PubMed]
- Marongiu F, Buggi F, Mingozzi M, et al. A rare case of primary necrotising fasciitis of the breast: combined use of hyperbaric oxygen and negative pressure wound therapy to conserve the breast. Review of literature. Int Wound J 2017;14:349-54. [Crossref] [PubMed]
- ALShareef B. ALSaleh N. Necrotizing Fasciitis of the Breast: Case Report with Literature Review. Case Rep Surg 2018;2018:1370680 [PubMed]
- Tena D, Saa L. Skin and soft tissue infection caused by Cutibacterium (formerly Propionibacterium) avidum: Report of eleven cases. Anaerobe 2019;56:91-4. [Crossref] [PubMed]
- Wong CH, Chang HC, Pasupathy S, et al. Necrotizing Fasciitis: Clinical Presentation, Microbiology, and Determinants of Mortality. J Bone Joint Surg Am 2003;85:1454-60. [Crossref] [PubMed]
- Wong CH, Khin LW, Heng KS, et al. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004;32:1535-41. [Crossref] [PubMed]
- Fontes RA Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg 2000;8:151-8. [Crossref] [PubMed]
- Şimşek G, Gündeş E, Tekin Ş, et al. Bilateral Breast Abscess Caused by E. coli in a Non-lactating Woman: A Rare Case. J Breast Health 2014;10:174-6. [Crossref] [PubMed]
Cite this article as: Stoeckl E, Dedhia PH, Wilke LG, Long KL. Necrotizing soft tissue infection of the breast: bilateral presentation in a male patient. Ann Breast Surg 2019;3:19.

