
Pathological and radiological assessments of Paget’s disease
Introduction
Paget’s disease (PD) of the nipple was first reported and described as “an eruption on the nipple and areola” by Sir James Paget in 1874 (1). Several studies have since provided evidence supporting the concept that PD is derived from an underlying adenocarcinoma (2). It is indeed an in situ disease with generally very favourable outcomes, and thus should be treated as an early breast cancer, not requiring systemic therapies after surgery.
However, this disease has long presented a dilemma for surgeons. Because PD is located in the centre of the breast, total mastectomy has been the first choice for treating PD. If a patient requests central lumpectomy (CL), deformity of the breast, caused by the surgery and subsequent radiation therapy, is inevitable. The presence of a co-existing intra-breast lesion is another challenging management issue. It has also been reported that invasive or in situ ductal carcinoma co-exists with PD in 82% to 94% of cases (3-6). These intra-breast lesions might exist multifocally and multicentrally, such that precise preoperative assessment by imaging is also important for selecting the surgical procedure. A guideline published by the National Comprehensive Cancer Network is available with an algorithm for diagnostic and surgical strategies for PD patients (7). However, there is an annotation stating that, “Mastectomy is always an option with any manifestation of PD”, reflecting the difficulty of surgically managing this disease.
The very low incidence rate of PD (0.6–2%) (2,8) might be a major factor impeding the basic and clinical studies needed to resolve problems with managing PD, including selection of the optimal surgical procedure. Thus, in the current study, we retrospectively investigated PD cases in our institution to clarify the details of this disease. We also endeavoured to identify the most appropriate surgical procedures.
Methods
Patients
Among 4,341 breast cancer cases, undergoing curative surgery during the 2008 through 2018 period at our institution, there were 29 with PD (0.7%). We excluded 12 of these 29 patients who clinically showed a mass lesion in the breast, based on palpation and imaging. We retrospectively investigated the remaining 17 cases, all Japanese women. This study was carried out with approval from the ethics committee of Juntendo University Hospital (No. 16-097) and all data were collected after obtaining informed consent from the 17 participating patients.
Pathological evaluation
Pathological examinations were carried out at Juntendo University Hospital by two experienced pathologists. Immunohistochemical assessment was conducted in the Paget cells. Oestrogen receptor (ER) and progesterone receptor (PgR) statuses were assessed semi-quantitatively and reported as positive when more than 1% of the nuclei of cancer cells showed staining. Epidermal growth factor receptor 2 (HER2) was judged to be positive when strong staining of the entire cell membrane was observed in >10% of tumour cells. As to Ki67, a hot spot was chosen under 200× magnification and cells positive for nuclear Ki67 were then counted. We employed 20% as the cut-off value for the Ki67 labeling index (L.I.), allowing patients to be divided into high and low groups.
Results
Surgical procedures and clinicopathological features of the 17 cases
Clinicopathological features of the 17 cases are shown in Table 1. All patients were female and mean age at diagnosis was 64 (range, 48–79) years. As to chief complaints, nipple eczema/erythema and nipple discharge were observed, respectively, in 13 (76%) and 4 (24%) patients. Skin biopsy of the nipple areolar complex was performed in 12 cases (71%) before surgery. Three patients underwent additional biopsy of suspected lesions in the breast. For 5 patients who were expected to have disease spread into the breast, based on imaging findings, needle biopsy of the intra-breast lesion was performed instead of skin biopsy.
Table 1
| Variables | Category | n [%] |
|---|---|---|
| Sex | Female | 17 [100] |
| Male | 0 [0] | |
| Age | <50 | 2 [12] |
| ≥50 | 15 [88] | |
| Symptom | Nipple eczema or erythema | 13 [76] |
| Nipple discharge | 4 [24] | |
| Surgical procedure for the breast | Bt* | 13 [76] |
| Bt + TE | 2 [12] | |
| CL | 2 [12] | |
| Histology | PD alone | 2 [12] |
| PD with DCIS | 10 [59] | |
| PD with IDC | 5 [29] | |
| Size of in situ tumour component, mean (range), (mm) | PD alone | 0 |
| PD with DCIS | 37 (range, 5–125) | |
| PD with IDC** | 65 (range, 30–95) | |
| Nodal status | Negative | 16 [94] |
| Positive | 1 [6] | |
| Surgical margin | Negative | 17 [100] |
| Positive | 0 [0] | |
| Tumour grade | Low/intermediate | 6 [46] |
| High | 7 [54] | |
| Unknown | 4 | |
| Ki67 L.I. | <20% | 2 [17] |
| ≥20% | 10 [83] | |
| Unknown | 5 | |
| Subtype | ER-positive/HER2-negative | 1 [6] |
| ER-positive/HER2-positive | 0 [0] | |
| ER-negative/HER2-positive | 13 [76] | |
| ER-negative/HER2-negative | 3 [18] |
*, including one patient for whom CL was planned but had to be switched to Bt intraoperatively; **, invasive component: 4 mm (range, 1–8 mm). Bt, total mastectomy; TE, tissue expander; CL, central lumpectomy; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; Ki67 L.I., Ki67 labelling index; PD, Paget’s disease.
As to operative procedures, total mastectomy (Bt) and CL were performed in 15 and 2 patients, respectively. Among the 15 patients undergoing Bt, two simultaneously received tissue expander implantation (TE). One patient initially requested CL but had to be switched to Bt because intraoperative pathological assessment of a frozen section revealed the surgical margin to be positive. Sentinel lymph node biopsy was performed for all patients and one, who had a positive lymph node, also underwent axillary lymph node dissection.
On final pathological diagnosis, two patients had PD alone (12%). PD with ductal carcinoma in situ (PD + DCIS) and invasive ductal carcinoma (PD + IDC) were observed in 10 (59%) and 5 (29%) patients, respectively. As for the extent of the in situ component, the mean diameter was 41 mm (range, 0–125 mm). When the mean size was compared between PD + DCIS (37 mm) and PD + IDC (65 mm), the latter was found to be larger, although the difference was not statistically significant by the two-sided Student’s t-test (P=0.16). The mean size of the invasive component in PD + IDC cases was 4 mm (1–8 mm). Surgical margins were negative in all patients. The rate of high tumour grade was 54% (7 cases) and high Ki67 L.I. was observed in 58% (7 cases). As to ER and HER2 statuses, ER-positive/HER2-negative, ER-positive/HER2-positive, ER-negative/HER2-positive and ER-negative/HER2-negative were observed in 6%, 0%, 76% and 18%, respectively, of patients.
Following surgery, 2 patients who underwent CL received radiation therapy to the conserved breast. Adjuvant chemotherapy was given to the patient who had axial lymph node metastasis but the other 16 patients received no systemic treatments, such as endocrine therapy. To date, no patient has developed either local recurrence or distant metastasis during the median 60-month observation period.
Imaging findings
All patients underwent MMG before surgery. MRI was performed in 14 cases (82%), based on the decisions made by the patient’s primary physicians. Relationships between imaging findings and pathological diagnosis are shown in Figure 1. Disease spread into the breast was pre-operatively suspected in 8 cases with micro-calcifications on MMG (47% of the 17 patients) and in 8 cases with segmental enhancement on MRI (57% of the 14 cases who underwent MRI). Patients with PD (n=2) had no remarkable findings on either examination (highlighted in yellow in Figure 1A,B).
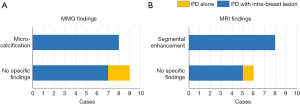
As to the accuracy of diagnosis based on the combination of MMG and MRI, we examined 14 patients who had undergone both imaging examinations (Table 2). Sensitivity and specificity for intra-breast disease were 62% (8 of 13) and 100% (1 of 1), respectively.
Table 2
| Specific findings on any modality | PD with intra-breast lesion | PD alone |
|---|---|---|
| Yes | 8 | 0 |
| No | 5 | 1 |
Sensitivity =8/13 (62%), specificity =1/1 (100%). PD, Paget’s disease.
Discussion
In the current study, 2 patients had PD alone, accounting for only 0.05% of all breast cancer cases at our institution in the 11 years since 2008. Also, the rate of PD alone was 12% of the 17 PD cases in this study without a mass lesion. Wong et al. reported the rate of PD alone to be 7% in an investigation of 2,631 PD cases during the period from 2000 to 2011 using Surveillance, Epidemiology and End Results (SEER) data (9). Meanwhile, 88% of our cases had intra-breast components. This rate is consistent with previous research by Onoe et al. (8). They investigated 27 PD cases during a 46-year period and found that 85% of these cases had a DCIS or an IDC component in the breast. Our data have reconfirmed how rare PD alone is and also provide a warning to surgeons regarding the possibility of intra-breast lesions being present.
There are a number of reports on breast conserving surgery for patients with PD (3,10,11). A prospective clinical study showed successful local control with breast conserving surgery followed by irradiation for PD + DCIS (12). When we initially choose CL, precise pre-operative evaluation of the intra-breast lesion with imaging is crucial. However, the sensitivity of imaging was only 62% in the current study. Although false negatives on MRI are widely recognized (13), this low rate raises suspicion that PD might tend to have ductal components, which would be undetectable by current imaging techniques.
In a systematic review, Helme et al. concluded that breast conserving surgery should be recommended for PD (14), although only 47% of PD alone cases underwent Bt (9) in the aforementioned SEER dataset. Onoe et al. indicated that maintaining acceptable cosmetic outcomes with CL is difficult in Japanese women (8). Considering the high frequency of extensive DCIS components in our patient group, from the viewpoint of cosmesis, recommending breast conserving surgery might not always suitable for Asian women, who have relatively small breasts, with PD. Further studies examining breast size are clearly needed to examine this issue in detail.
As to the PD subtype, the ER-negative/HER2-positive rate was high (76%), consistent with previous reports (8,15). Why this disease is frequently of the HER2-positive is not fully understood. It is, however, interesting that in our dataset the invasive component was relatively small as compared to the widespread in situ component, despite HER2 overexpression reportedly being a risk factor for invasion in DCIS (16,17). The biological significance of HER2 overexpression in PD is a subject that merits future investigation.
Our data confirm that breast conserving surgery may be difficult for patients with PD. Even when a patient with PD requests breast conserving surgery, the surgical procedure might have to be determined in consideration of a balance between curability and cosmesis.
Acknowledgments
We appreciate Dr. Bierta Barfod for her help with the language editing.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.05.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was carried out with approval from the ethics committee of Juntendo University Hospital (No. 16-097) and all data were collected after obtaining informed consent from the 17 participating patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Paget J. On disease of the mammary areola preceding cancer of the mammary gland. St. Bartholomew's Hospital Reports 1874;10:87-9.
- Rosen PP. Paget's disease of the nipple. Rosen's Breast Pathology. 3rd ed. Philadelphia, PA: Lippincott-Williams & Wilkins, 2009:621-35.
- Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998;187:171-7. [Crossref] [PubMed]
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: Changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer 2006;107:1448-58. [Crossref] [PubMed]
- Yim JH, Wick MR, Philpott GW, et al. Underlying pathology in mammary Paget's disease. Ann Surg Oncol 1997;4:287-92. [Crossref] [PubMed]
- Dominici LS, Lester S, Liao G-S, et al. Current surgical approach to Paget's disease. Am J Surg 2012;204:18-22. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practive Guidelines in Oncology. In: Breast Cancer. 2018. Available online: https://www.nccn.org/patients/
- Onoe S, Kinoshita T, Tamura N, et al. Feasibility of breast conserving surgery for Paget’s disease. Breast 2011;20:515-8. [Crossref] [PubMed]
- Wong SM, Freedman RA, Stamell E, et al. Modern Trends in the Surgical Management of Paget’s Disease. Ann Surg Oncol 2015;22:3308-16. [Crossref] [PubMed]
- Pierce LJ, Haffty BG, Solin LJ, et al. The conservative management of Paget's disease of the breast with radiotherapy. Cancer 1997;80:1065-72. [Crossref] [PubMed]
- Singh A, Sutton RJ, Baker CB, et al. Is mastectomy overtreatment for Paget's disease of the nipple? Breast 1999;8:191-4. [Crossref] [PubMed]
- Bijker N, Rutgers EJT, Duchateau L, et al. Breast-conserving therapy for paget disease of the nipple. Cancer 2001;91:472-7. [Crossref] [PubMed]
- Frei KA, Bonel HM, Pelte MF, et al. Paget Disease of the Breast: Findings at Magnetic Resonance Imaging and Histopathologic Correlation. Invest Radiol 2005;40:363-7. [Crossref] [PubMed]
- Helme S, Harvey K, Agrawal A. Breast-conserving surgery in patients with Paget's disease. Br J Surg 2015;102:1167-74. [Crossref] [PubMed]
- Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat 2013;141:1-12. [Crossref] [PubMed]
- Borgquist S, Zhou W, Jirström K, et al. The prognostic role of HER2 expression in ductal breast carcinoma in situ (DCIS); a population-based cohort study. BMC Cancer 2015;15:468. [Crossref] [PubMed]
- Horimoto Y, Tokuda E, Arakawa A, et al. Significance of HER2 protein examination in ductal carcinoma in situ. J Surg Res 2011;167:e205-10. [Crossref] [PubMed]
Cite this article as: Nakai K, Horimoto Y, Semba R, Arakawa A, Saito M. Pathological and radiological assessments of Paget’s disease. Ann Breast Surg 2019;3:11.

