
Maximum standardized uptake value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography could replace pathological diagnosis in luminal breast cancer
Introduction
Breast cancer is divided into several intrinsic subtypes: luminal type, human epidermal growth factor receptor 2 (HER2)-enriched type, and triple negative type based on gene expression profiling (1). Luminal-type breast cancer is hormone receptor-positive and HER2-negative. The indication for chemotherapy of luminal breast cancer is more complicated than that for HER2-enriched and triple negative breast cancers.
18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) is as an oncological imaging tool for diagnosing, staging, and determining treatment response (2). The maximum standardized uptake value (SUVmax) measures glucose metabolism, which reflects metabolic activity of tumor; it is also useful for predicting malignant behavior and prognosis of breast cancer (3). Moreover, the Ki-67 labeling index (LI) is another method for determining cancer aggressiveness; Ki-67 is a proliferation-related antigen, and a high Ki-67 LI is associated with more aggressive cancers.
This study aimed to determine whether SUVmax of PET/CT could replace pathological diagnosis in luminal breast cancer. Accordingly, we investigated (I) the relationship of SUVmax with Ki-67 LI and other clinicopathological features in luminal-type breast cancer and (II) whether SUVmax can help indicate the appropriateness of adjuvant chemotherapy for luminal-type breast cancer.
Methods
Patients
This retrospective study enrolled 85 patients with estrogen receptor (ER)-positive, HER2-negative (luminal-type) breast cancer between August 2013 and January 2018 who underwent 18F-FDG PET/CT scans and had not received any treatment before surgery at Fujisawa City Hospital. Patients with stage IV cancer were excluded. Moreover, to avoid the potential influence of needle biopsy-induced inflammation, needle biopsy and PET/CT were performed with an interval of at least 2 weeks. This study was approved by the Fujisawa City Hospital Ethics Committee (F2017039).
18F-FDG PET/CT
18F-FDG PET/CT scans were performed at Fujisawa City Hospital. The patients ate at least 6 h before the PET/CT examination. Each patient’s blood glucose level was measured to ensure that it did not exceed 150 mg/dL. Ninety minutes after intravenously administering 4 Bq/kg 18F-FDG, a whole-body scan was performed. Images were obtained using a PET/CT scanner (GEMINI TF, Philips). A transmission scan using CT for attenuation correction and anatomical imaging was acquired for 30 s. An intravenous contrast agent was not administered to patients for the CT portion of the 18F-FDG PET/CT.
PET/CT images were analyzed by two experienced radiologists. They calculated SUVmax, which is the SUVmax, as determined by the pixel density within the region of interest.
Histological analysis
Tumor histology and biology using tissue samples obtained by needle biopsy or during surgery were evaluated by two experienced pathologists. Immunohistochemical staining was performed using anti-ER antibodies (clone SP1: Roche Diagnostics, North America), anti-progesterone receptor (PgR) antibodies (clone 1E2: Roche Diagnostics, North America), anti-HER2 antibodies (clone 4B5: Roche Diagnostics, North America), and anti-Ki-67 antibodies (clone MIB-1: Dako Cytomation; Carpinteria, CA, USA). ER and PgR positivity were defined as positive immunohistochemical staining of at least 10% of the tumor cells for ER and PgR, respectively. HER2 positivity was defined as positive when more than 30% of the cells showed positive immunohistochemical staining. Ki-67 LI, which is the percentage of positive nuclear immunostaining cells, was calculated. Several sites were randomly selected, including the areas exhibiting the highest level of immunostaining. Ki-67 LI of the most densely labelled areas was used for analysis.
Indication for adjuvant chemotherapy
At our hospital, the indication for adjuvant chemotherapy for luminal-type breast cancer during the study period was as follows: PgR negative, Ki-67 LI >14%, nuclear grade 3, or lymph node metastasis-positive. If any of these were satisfied, it was regarded as an indication for adjuvant chemotherapy in luminal breast cancer.
Statistical analysis
Statistical analyses were performed using the Statistical Program for Social Sciences software (version 20.0, SPSS Inc., Chicago, USA). All tests were two-sided, and P<0.05 was considered significant. To investigate the correlation of SUVmax with tumor size and Ki-67 LI, Pearson’s product moment correlation coefficient (r) was calculated. A receiver operating characteristic (ROC) curve was created to investigate the SUVmax cutoff value that predicted an indication for adjuvant chemotherapy, and the area under the curve was calculated.
Results
Patient and tumor characteristics
Patient and tumor characteristics are shown in Table 1. The median age was 59 years (range, 37–85 years). The median tumor size was 20 mm (range, 6–70 mm). Seventeen patients (20%) were positive for lymph node metastasis, but no patients exhibited distant metastasis. All patients were ER-positive and HER2-negative, and 71 patients (84%) were PgR-positive. The median SUVmax was 4.41 (range, 1.00–14.00), and 18F-FDG uptake to the tumor was observed in all patients.
Table 1
| Characteristics | Number |
|---|---|
| Age (years) [range] | 59 [37–85] |
| Histological type | |
| Invasive ductal carcinoma | 72 |
| Special types | 13 |
| Tumor size (mm) [range] | 20 [6–70] |
| Lymph node status | |
| Present | 17 |
| Absent | 68 |
| Distant metastasis | |
| Present | 0 |
| Absent | 85 |
| Nuclear grade | |
| 1 | 63 |
| 2 | 20 |
| 3 | 2 |
| Lymphatic invasion | |
| Present | 12 |
| Absent | 73 |
| Vascular invasion | |
| Present | 4 |
| Absent | 81 |
| Estrogen receptor-positivity | |
| Present | 85 |
| Absent | 0 |
| Progesterone receptor-positivity | |
| Present | 71 |
| Absent | 14 |
| Human epidermal growth factor receptor 2-positivity | |
| Present | 0 |
| Absent | 85 |
| Ki-67 labeling index [range] | 16 [1–60] |
| Maximum standardized uptake [range] | 4.41 [1.00–14.00] |
Correlation between SUVmax and tumor characteristics
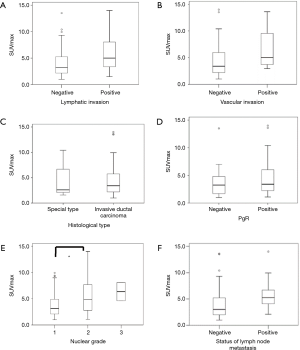
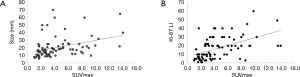
Figure 1 shows the association between SUVmax and clinicopathological features. SUVmax significantly differed between the positive and negative groups that showed lymphatic invasion (P=0.018), nuclear grade (P=0.019), and lymph node metastasis (P=0.035). There was a tendency toward correlation between SUVmax and tumor size (r=0.365, P=0.001; Figure 2A). Moreover, a significant correlation was observed between SUVmax and Ki-67 LI (r=0.516, P<0.0001; Figure 2B). However, SUVmax was not significantly different between the positive and negative groups that showed vascular invasion (P=0.128), histological type (P=0.936), or PgR status (P=0.689).
Prediction of adjuvant chemotherapy indication
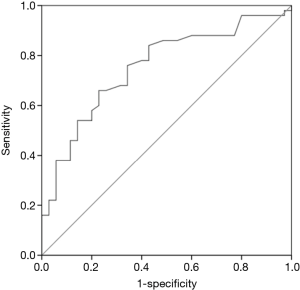
To investigate whether SUVmax can help indicate the appropriateness of adjuvant chemotherapy for luminal-type breast cancer in accordance with guidelines of our hospital, we used SUVmax values to construct a ROC curve. The area under the ROC curve was 0.753; at a cutoff SUVmax of 3.51 (Figure 3), the sensitivity was 66.0%, and specificity was 73.1%.
Discussion
We investigated correlations between SUVmax and clinicopathological features in luminal-type breast cancer. SUVmax was correlated with lymphatic invasion, nuclear grade, lymph node metastasis, and Ki-67 LI.
Regarding the correlation with Ki-67 LI, a meta-analysis suggested that SUVmax was correlated with Ki-67 LI in various cancers (4), with a correlation coefficient of 0.29–0.56 for breast cancer (5-7). However, in these prior studies, no distinction between ER-positive and ER-negative breast cancer was observed. Cheng et al. (7) reported a correlation coefficient of 0.23 between SUVmax and Ki-67 LI in ER-positive breast cancer compared with a value of 0.67 in the present study. The difference between the correlation values in Cheng et al.’s report and the present study could be because of the difference in the number of patients enrolled in each study. Moreover, Cheng et al.’s study included patients with stage IV breast cancer, while our study did not. Therefore, if analyses were limited to luminal breast cancer while excluding stage IV breast cancer, Ki-67 LI could be correlated with SUVmax. In contrast, as we previously reported (8), Ki-67 LI values are slightly variable. Depending on the tissue used (either from needle biopsy or taken during surgery) and the site of tissue to be evaluated, Ki-67 LI varied; furthermore, Ki-67 LI also differed among facilities. Therefore, analyzing the correlation between Ki-67 LI and SUVmax has some limitations.
To provide individualized medical treatment for breast cancer, considerable efforts regarding immunostaining are required (9). Indications for adjuvant chemotherapy for luminal-type breast cancer are difficult to determine, with no defined consensus yet. The 12th St. Gallen Consensus Conference regarding early breast cancer announced that the status of PgR, Ki-67, lymph node metastasis, or nuclear grade should be used as indications for adjuvant chemotherapy. This can be a serious problem in countries, such as Japan, where there is an insufficient number of pathologists. Therefore, it can be beneficial to use imaging diagnostics in lieu of immunostaining. In Japan, there are only 1.0 pathological specialists per 100,000 population, or 0.5% of all doctors. This is only 20% of the relative number compared to that in the US. Because of this limitation, we examined whether imaging diagnosis could replace pathological diagnosis such as Ki-67 LI, PgR status, or nuclear grade. Therefore, we investigated whether SUVmax could be used to help classify patients with and without an indication for adjuvant chemotherapy. When we constructed a ROC curve depending on SUVmax, the area under the curve was 0.753, and at a cutoff SUVmax of 3.51, the sensitivity and specificity were 66.0% and 73.1%, respectively. Therefore, imaging diagnosis was useful, but the sensitivity and specificity were not satisfactory; thus, further improvement is necessary. For example, Yamane et al. (10) reported that findings on 18F-fluorothymidine PET showed better correlation with the immunohistochemical index of cell proliferation in leiomyoma compared with those on 18F-FDG PET. In addition, because SUV is related to cell density, analyses should be performed based on histological type. However, this was not possible in the present study owing to the small sample size. Moreover, SUV is affected by tumor size. Because of the partial volume effect, it is not possible to obtain an accurate count in a small lesion, and the SUV is underestimated. Calculating the recovery count in the phantom experiment makes it possible to correct the partial volume effect. However, we do not employ phantom experiments in our facility. Therefore, the use of phantom experiments to assist with obtaining more accurate SUV measurements remains to be explored.
In recent years, a method to determine the degree of malignancy in breast cancer based on genetic assays, such as the 21-gene recurrence score, has been developed; however, this is not yet available at our hospital, and thus, the indication for chemotherapy depends only on pathological information only. Therefore, for luminal-type breast cancer, the indication included PgR negativity, Ki-67 LI >14%, nuclear grade of 3, or lymph node metastasis-positive.
In our study, the SUVmax cutoff value of indication for chemotherapy was set to 3.5. On the other hand, Ahn et al. (11) previously reported that in ER-positive, HER2-negative breast cancer, SUVmax ≥4 was independently associated with a 21-gene recurrence score ≥26, which was considered as a recommendation to undergo adjuvant chemotherapy. In addition, a study by Kitajima et al. (12) reported the prognostic value of SUVmax in breast cancer using a cutoff SUVmax of 3.6; an SUVmax of 3.5–4 was considered to indicate highly malignant breast cancer.
This study had several limitations. First, the study population was small, and the study design was retrospective. A larger study population and additional outcome data are needed to confirm our results. Second, the usefulness of metabolic tumor volume and total lesion glycolysis have been reported (13); however, these are not calculated at our hospital. Third, the relationship between SUVmax and prognosis is not clear, because no patient showed relapse due to the short duration of follow-up.
In conclusion, SUVmax of 18F-FDG PET could replace pathological diagnosis in luminal breast cancer.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.02.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Fujisawa City Hospital Ethics Committee (F2017039). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology 2004;231:305-32. [Crossref] [PubMed]
- Zhang J, Jia Z, Ragaz J, et al. The maximum standardized uptake value of 18 F-FDG PET scan to determine prognosis of hormone-receptor positive metastatic breast cancer. BMC Cancer 2013;13:42. [Crossref] [PubMed]
- Deng SM, Zhang W, Zhang B, et al. Correlation between the Uptake of 18F-Fluorodeoxyglucose (18F-FDG) and the Expression of Proliferation-Associated Antigen Ki-67 in Cancer Patients: A Meta-Analysis. PLoS One 2015;10:e0129028 [Crossref] [PubMed]
- Koolen BB, Vrancken Peeters MJ, et al. Association of primary tumour FDG uptake with clinical, histopathological and molecular characteristics in breast cancer patients scheduled for neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging 2012;39:1830-8. [Crossref] [PubMed]
- Kajáry K, Tőkés T, Dank M, et al. Correlation of the value of 18F-FDG uptake, described by SUVmax, SUVavg, metabolic tumour volume and total lesion glycolysis, to clinicopathological prognostic factors and biological subtypes in breast cancer. Nucl Med Commun 2015;36:28-37. [Crossref] [PubMed]
- Cheng J, Lei L, Xu J, et al. 18F-fluoromisonidazole PET/CT: a potential tool for predicting primary endocrine therapy resistance in breast cancer. J Nucl Med 2013;54:333-40. [Crossref] [PubMed]
- Yamamoto S, Chishima T, Mastubara Y, et al. Variability in measuring the Ki-67 labeling index in patients with breast cancer. Clin Breast Cancer 2015;15:e35-9. [Crossref] [PubMed]
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533-46. [Crossref] [PubMed]
- Yamane T, Takaoka A, Kita M, et al. 18F-FLT PET performs better than 18F-FDG PET in differentiating malignant uterine corpus tumors from benign leiomyoma. Ann Nucl Med 2012;26:478-84. [Crossref] [PubMed]
- Ahn SG, Lee JH, Lee HW, et al. Comparison of standardized uptake value of 18F-FDG-PET-CT with 21-gene recurrence score in estrogen receptor-positive, HER2-negative breast cancer. PLoS One 2017;12:e0175048 [Crossref] [PubMed]
- Kitajima K, Miyoshi Y, Yamano T, et al. Prognostic value of FDG-PET and DWI in breast cancer. Ann Nucl Med 2018;32:44-53. [Crossref] [PubMed]
- Takahashi N, Yamamoto T, Matsushita H, et al. Metabolic tumor volume on FDG-PET/CT is a possible prognostic factor for Stage I lung cancer patients treated with stereotactic body radiation therapy: a retrospective clinical study. J Radiat Res 2016;57:655-61. [Crossref] [PubMed]
Cite this article as: Yamamoto S, Yamagishi S, Kohno T, Tajiri R, Gondo T, Fujii Y, Tsukamoto H. Maximum standardized uptake value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography could replace pathological diagnosis in luminal breast cancer. Ann Breast Surg 2019;3:5.

