
Factors influencing post-mastectomy reconstruction in breast cancer patients aged 40 years and younger
Highlight box
Key findings
• Contralateral prophylactic mastectomy (CPM) is performed in over half of women ≤40 years regardless of whether they undergo reconstruction.
• Private insurance, higher income, and CPM are all positively associated with post-mastectomy reconstruction.
• Pathologic complete response to neoadjuvant chemotherapy was associated with increased rates of post-mastectomy reconstruction.
What is known and what is new?
• Young women with breast cancer are more likely to undergo post-mastectomy reconstruction than women >40 years.
• Post-mastectomy reconstruction is predicted by similar demographic factors in young women and women >40 years.
• In young women, more advanced disease was associated with decreased rates of post-mastectomy reconstruction.
• CPM remains common in young women despite a lack of evidence for improved survival.
What is the implication, and what should change now?
• Breast surgeons should ensure that all women are appropriately counselled on their reconstruction options and associated risks and benefits.
Introduction
Approximately 1 in 8 women will be diagnosed with breast cancer during their lifetime. Young women make up a small but important portion of this group. Approximately 6% of breast cancers in the United States occur in women <40 years old, a significant finding considering screening mammography is recommended to begin at age 40 years for an average risk woman (1). Breast cancer patients age ≤40 years are more often diagnosed with advanced disease and aggressive subtypes such as triple negative breast cancer (TNBC), human epidermal growth factor receptor 2 positive (HER2+) breast cancer, and inflammatory breast cancer (2-5). Young age at breast cancer diagnosis is associated with poorer outcomes such as disease recurrence and mortality, and breast cancer is the leading cause of cancer-related deaths in women ≤40 years (2,3,6-9).
Previous studies have established that young women with breast cancer are more likely to undergo mastectomy and mastectomy with reconstruction (MR) compared to women >40 years (6,7,10,11), with reconstruction rates reaching 89% in the multicenter Young Women’s Breast Cancer Study (12). Rates of contralateral prophylactic mastectomy (CPM) are also higher in breast cancer patients ≤40 years, and have increased over time, mirroring increasing use of MR (6,11,13). These trends persist despite an increasing body of compelling evidence demonstrating that women—and particularly young women—who undergo breast conserving therapy (BCT) have superior survival outcomes (14-17), and despite well-established literature showing lack of survival benefit for CPM in average-risk women with unilateral breast cancer (18).
Many factors influence whether patients undergo MR, including demographic and disease characteristics as well as use of adjuvant therapy, particularly radiation (19-22). Prior research has identified persistent socioeconomic and geographic disparities in the receipt of MR, with non-White race, public insurance, and receiving care in the South or West regions of the United States all associated with lower rates of MR (20,21). However, in these studies without age stratification, young women are likely not adequately represented due to the relatively low incidence of breast cancer in this population. Several retrospective cohort studies specifically examining patients >65 years have shown that younger age, White race, lower co-morbidity score, and treatment at academic centers are positively associated with MR (19,22,23). However, there is a paucity of high-quality population-level research focusing on these questions in women age ≤40 years with newly diagnosed breast cancer (12,24).
Utilizing the National Cancer Database (NCDB), we aimed to identify factors influencing MR in female patients aged ≤40 years with unilateral invasive breast cancer. In addition, we examined whether the factors associated with MR were predictors of CPM, and which patient, tumor, and treatment variables were associated with autologous vs. implant-based reconstruction. Finally, we determined the relationship of MR and CPM with overall survival (OS). It is our hope that our findings will help breast oncology providers and patients better understand the complexities of surgical decision-making in young women with breast cancer and address disparities in post-mastectomy care in this unique population. We present this article in accordance with the STROBE reporting checklist (https://abs.amegroups.com/article/view/10.21037/abs-23-48/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). After Institutional Review Board exemption due to the deidentified data source, a retrospective cohort study using the NCDB was performed. Jointly sponsored by the American College of Surgeons and the American Cancer Society, the NCDB is a clinical oncology database sourced from hospital registry data that represents >70% of newly diagnosed cancer cases nationwide, and 1,500 Commission on Cancer (CoC)-accredited facilities. Definitions of the database variables are available from the dictionary of NCDB Participant Use Data File (http://ncdbpuf.facs.org). The CoC’s NCDB and the hospitals participating in the CoC NCDB are the sources of the de-identified data used herein: they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Female patients aged ≤40 years with American Joint Committee on Cancer (AJCC) clinical stage I–III unilateral invasive breast cancer treated with mastectomy {subcutaneous [nipple-sparing (NSM)], simple (total), modified radical} from 2010–2018 were included (Figure 1). Simple mastectomy in the NCDB includes skin-sparing mastectomy for patients who underwent reconstruction. Patients who did not have surgery, underwent partial mastectomy or radical mastectomy, or had AJCC clinical stage IV disease were excluded. Bilateral disease was excluded to ensure all included bilateral mastectomies were also CPM. Invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), and other primary breast malignancies were included (histology codes 8500, 8521, 8523, 8525, 8541, 8543, 8520, 8524, 8522, 8502, 8575, 8343, 8260, 8453, 8510, 8512, 8513) (http://ncdbpuf.facs.org).

Patients were stratified by receipt of reconstruction: mastectomy alone (MA) and MR. Reconstruction was further categorized as autologous, implant-based, or combined-type (implant and autologous). The NCDB codes reconstruction as part of the first course of treatment regardless of whether performed at the time of mastectomy (immediate) or delayed. Patient characteristics (age, race, Charlson-Deyo comorbidity score, insurance status, household income, education level, distance to facility), facility information (type, community designation), tumor factors (AJCC 7th edition clinical stage and pathologic stage, histologic grade, hormone receptor status), and treatment information (breast and axillary surgery, reconstruction, chemotherapy, endocrine therapy, radiation therapy, sequence of treatment, length of stay, 30-day readmission rates) were collected. Patients were considered hormone receptor positive (HR+) if either or both of the estrogen or progesterone receptors were positive. Facility type and location were unavailable because the NCDB suppresses this data for patients <40 due to low case counts. The 2012 definitions of “Scope of Regional Lymph Node Surgery”, “Percent No High School Degree Quartiles” and “Median Income Quartiles” were applied to the dataset, resulting in exclusion of these variables for patients from 2010–2011. The two lowest (<$40,227 and $40,227–$50,353) and two highest ($50,354–$63,332, ≥$63,333) median income quartiles were combined for simplicity of analysis. Axillary lymph node dissection (ALND) is considered removal of greater than three lymph nodes based on coding for the “Scope of Regional Lymph Node Surgery” variable.
Chi-squared analyses evaluated differences among the cohorts for patient, tumor, and treatment characteristics. Multivariable logistic regression identified factors associated with MR, reconstruction type, and CPM. Patients designated in the NCDB as receiving combined-type reconstruction were included in the autologous group for this analysis. Kaplan-Meier survival estimates compared 5-year OS, and multivariable Cox proportional hazards regression identified factors associated with OS. A subset analysis of patients who underwent neoadjuvant chemotherapy (NAC) was performed to determine if treatment response was associated with MR or CPM. Those who received NAC or both neoadjuvant and adjuvant chemotherapy were included in this analysis. Downstage, defined by pathologic stage less than clinical stage for both tumor (T) and nodal (N) response; and upstage, classified as pathologic stage greater than clinical stage, were compared between groups. Pathologic complete response (pCR) was considered pT0N0 and those with cN0 disease were excluded from calculations for N response. All statistical analysis was performed using STATA SE Version 17.0.
Results
Demographics and clinical characteristics
Of the 43,065 patients who met inclusion criteria, 28,508 (66%) underwent MR and 14,557 (34%) underwent MA. Mean age was not different between groups (35.3 years MR vs. 35.4 years MA). Non-Hispanic Black and Hispanic patients comprised a greater proportion of the MA group (Table 1). Significantly more patients in the MR group had private insurance (83.8% MR vs. 68.5% MA, P<0.001), higher annual income (≥$50,354, 71.5% MR vs. 57.1% MA, P<0.001), and higher levels of education. More MR patients had cT1 tumors (42.6% MR vs. 29.5% MA, P<0.001) and over two-thirds were clinically node-negative (67.8% MR vs. 51.1% MA, P<0.001). Poorly differentiated tumors (51.1% MR vs. 56.6% MA, P<0.001) and TNBC (18.6% MR vs. 22.1% MA, P<0.001) were more common in the MA group. AJCC pathologic stage was also higher in the MA group compared to MR (stage III 11.8% MR vs. 23.0% MA).
Table 1
| Characteristics | MA (n=14,557) | MR (n=28,508) | P value |
|---|---|---|---|
| Age, years, mean (± standard deviation) | 35.4 (±4.03) | 35.3 (±4.12) | 0.657 |
| Race | <0.001 | ||
| Non-Hispanic White | 8,811 (60.5) | 19,934 (69.9) | |
| Non-Hispanic Black | 2,382 (16.4) | 3,463 (12.2) | |
| Hispanic | 1,862 (12.8) | 2,541 (8.9) | |
| Other | 1,502 (10.3) | 2,570 (9.0) | |
| Charlson-Deyo co-morbidity score | <0.001 | ||
| 0 | 13,569 (93.2) | 26,649 (93.5) | |
| 1 | 868 (6.0) | 1,713 (6.0) | |
| ≥2 | 120 (0.8) | 146 (0.5) | |
| Insurance status | <0.001 | ||
| None | 923 (6.4) | 577 (2.1) | |
| Private | 9,850 (68.5) | 23,654 (83.8) | |
| Public (Medicare/Medicaid) | 3,608 (25.1) | 3,982 (14.1) | |
| Income† | <0.001 | ||
| ≤$50,353 | 5,486 (42.9) | 7,004 (28.5) | |
| ≥$50,354 | 7,289 (57.1) | 17,556 (71.5) | |
| Education level‡ | <0.001 | ||
| ≥13% | 6,037 (46.8) | 8,535 (34.5) | |
| <13% | 6,855 (53.2) | 16,178 (65.5) | |
| Community | <0.001 | ||
| Metro | 11,980 (84.3) | 24,799 (89.8) | |
| Urban | 1,999 (14.1) | 2,555 (9.3) | |
| Rural | 231 (1.6) | 249 (0.9) | |
| Distance to facility | <0.001 | ||
| ≤10 miles | 6,201 (42.6) | 11,662 (40.9) | |
| 11–50 miles | 5,516 (37.9) | 10,732 (37.6) | |
| >50 miles | 2,840 (19.5) | 6,114 (21.5) | |
| Tumor histology | 0.001 | ||
| IDC | 13,334 (91.6) | 26,102 (91.6) | |
| ILC | 531 (3.6) | 1,010 (3.5) | |
| IDC and ILC | 518 (3.6) | 1,147 (4.0) | |
| Other | 174 (1.2) | 249 (0.9) | |
| HR status | <0.001 | ||
| HR+/HER2− | 7,035 (48.4) | 15,093 (52.9) | |
| HR+/HER2+ | 2,550 (17.5) | 5,131 (18.0) | |
| HR−/HER2+ | 1,120 (7.7) | 1,855 (6.5) | |
| HR−/HER2− | 3,220 (22.1) | 5,292 (18.6) | |
| Unknown | 628 (4.3) | 1,135 (4.0) | |
| Grade | <0.001 | ||
| I: well differentiated | 863 (5.9) | 2,234 (7.8) | |
| II: moderately differentiated | 4,585 (31.5) | 10,210 (35.8) | |
| III: poorly differentiated | 8,233 (56.6) | 14,575 (51.1) | |
| IV: undifferentiated | 53 (0.4) | 54 (0.2) | |
| Unknown | 820 (5.6) | 1,434 (5.0) | |
| Clinical T stage | <0.001 | ||
| 1 | 4,283 (29.5) | 12,111 (42.6) | |
| 2 | 6,403 (44.1) | 12,390 (43.6) | |
| 3 | 2,733 (18.8) | 3,325 (11.7) | |
| 4 | 1,045 (7.2) | 527 (1.8) | |
| Unknown | 51 (0.4) | 66 (0.2) | |
| Clinical N stage | <0.001 | ||
| 0 | 7,407 (51.1) | 19,269 (67.8) | |
| 1 | 5,370 (37.0) | 7,569 (26.7) | |
| 2 | 919 (6.3) | 876 (3.1) | |
| 3 | 703 (4.9) | 556 (1.9) | |
| Unknown | 104 (0.7) | 136 (0.5) | |
| AJCC clinical stage | <0.001 | ||
| I | 3,354 (23.0) | 10,532 (36.9) | |
| II | 7,381 (50.7) | 14,341 (50.3) | |
| III | 3,822 (26.3) | 3,635 (12.8) | |
| Pathologic T stage | <0.001 | ||
| 0 | 1,863 (13.1) | 4,100 (14.7) | |
| 1 | 5,180 (36.5) | 13,412 (48.0) | |
| 2 | 4,811 (33.9) | 8,175 (29.2) | |
| 3 | 1,580 (11.1) | 1,538 (5.5) | |
| 4 | 305 (2.2) | 115 (0.4) | |
| Unknown | 450 (3.2) | 605 (2.2) | |
| Pathologic N stage | <0.001 | ||
| 0 | 6,665 (46.9) | 17,167 (61.4) | |
| 1 | 4,562 (32.1) | 7,733 (27.6) | |
| 2 | 1,803 (12.7) | 2,093 (7.5) | |
| 3 | 883 (6.2) | 651 (2.3) | |
| Unknown | 289 (2.0) | 339 (1.2) | |
| Pathologic M stage | <0.001 | ||
| 0 | 3,862 (98.2) | 8,872 (99.2) | |
| 1 | 72 (1.8) | 71 (0.8) | |
| Unknown | 0 (0.0) | 1 (0.0) | |
| AJCC pathologic stage | <0.001 | ||
| 0 | 509 (3.6) | 1,053 (3.8) | |
| I | 3,181 (22.3) | 9,874 (35.2) | |
| II | 5,337 (37.5) | 10,123 (26.0) | |
| III | 3,268 (23.0) | 3,317 (11.8) | |
| IV | 75 (0.5) | 69 (0.2) | |
| Unknown | 1,869 (13.1) | 3,645 (13.0) |
Data are shown as n (%) unless otherwise stated. †, median income quartiles—matches patient zip code with median income in the zip code, excludes patients before 2012; ‡, percent no high school degree 2012—matches patient zip code against the proportion of adults in the zip code that did not graduate high school, excludes patients before 2012. NCDB, National Cancer Database; MA, mastectomy alone; MR, mastectomy with reconstruction; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; AJCC, American Joint Committee on Cancer.
Treatment characteristics
MR patients less frequently received chemotherapy (82.9% MR vs. 90.1% MA, P<0.001), including NAC (Table 2). Receipt of endocrine therapy was higher in the MR group, mirroring HR status. ALND was more commonly performed in the MA group (61.8% MA vs. 44.6% MR, P<0.001). The majority of patients in both groups underwent CPM (60.6% MR vs. 50.9% MA, P<0.001). Nearly a quarter of patients in the MA group received modified radical mastectomy compared with a small number of cases in the MR group (24.3% MA vs. 7.4% MR, P<0.001). Rates of hospital readmission within 30 days of surgery were low and similar between groups (Table 2). Radiation was administered to half of patients in the MA group compared with only one-third in the MR group (54.3% MA vs. 37.4% MR, P<0.001).
Table 2
| Characteristics | MA (n=14,557) | MR (n=28,508) | P value |
|---|---|---|---|
| Chemotherapy sequence | <0.001 | ||
| None | 1,433 (9.9) | 4,836 (17.1) | |
| Neoadjuvant† | 5,681 (39.2) | 8,999 (31.7) | |
| Adjuvant | 5,871 (40.5) | 11,115 (39.2) | |
| Unknown sequence | 1,504 (10.4) | 3,393 (12.0) | |
| Endocrine therapy | <0.001 | ||
| None | 5,472 (37.6) | 9,241 (32.4) | |
| Endocrine therapy | 8,786 (60.4) | 18,755 (65.8) | |
| Unknown | 299 (2.0) | 512 (1.8) | |
| Immunotherapy | <0.001 | ||
| None | 12,198 (83.8) | 23,414 (82.1) | |
| Immunotherapy | 2326 (16.0) | 5,031 (17.7) | |
| Unknown | 33 (0.2) | 62 (0.2) | |
| Receipt of radiation | <0.001 | ||
| None | 6,260 (43.1) | 17,091 (60.0) | |
| Radiation | 7,888 (54.3) | 10,667 (37.4) | |
| Unknown | 385 (2.6) | 726 (2.6) | |
| Breast surgery | <0.001 | ||
| Simple mastectomy | 3,608 (24.8) | 5,666 (19.9) | |
| Nipple sparing mastectomy | 0 (0) | 3,450 (12.1) | |
| Modified radical mastectomy | 3,537 (24.3) | 2,100 (7.4) | |
| Contralateral prophylactic mastectomy | 7,412 (50.9) | 17,292 (60.6) | |
| Axillary surgery | <0.001 | ||
| None | 154 (1.4) | 268 (1.1) | |
| Sentinel lymph node biopsy | 3,964 (36.4) | 12,505 (54.0) | |
| Axillary lymph node dissection | 6,738 (61.8) | 10,343 (44.6) | |
| Unknown | 43 (0.4) | 64 (0.3) | |
| Length of hospital stay, days | <0.001 | ||
| 0 | 3,153 (21.7) | 4,632 (16.3) | |
| 1 | 6,237 (42.8) | 10,353 (36.3) | |
| 2 | 2,076 (14.3) | 6,894 (24.2) | |
| 3 | 503 (3.5) | 2,004 (7.0) | |
| 4 | 177 (1.2) | 1,033 (3.6) | |
| ≥5 | 2,411 (16.5) | 3,592 (12.6) | |
| Readmission within 30 days of surgery | 0.265 | ||
| No readmission | 13,787 (96.5) | 27,010 (96.3) | |
| Readmitted | 500 (3.5) | 1,042 (3.7) |
Data are shown as n (%). †, those undergoing neoadjuvant chemotherapy and adjuvant chemotherapy were included in the neoadjuvant chemotherapy group. NCDB, National Cancer Database; MA, mastectomy alone; MR, mastectomy with reconstruction.
Factors associated with post-mastectomy reconstruction
More recent treatment year (OR 1.06), higher income (OR 1.54), higher cT stage (OR 1.60), and CPM (OR 1.27, all P<0.001) (Table 3) were all positively associated with MR. Patients with TNBC, high grade tumors, and cN+ disease were each significantly less likely to undergo MR. Post-mastectomy radiation was associated with 25% reduced likelihood of MR (OR 0.75, P<0.001), and patients treated with adjuvant chemotherapy were also less likely to have MR (OR 0.87, P=0.009).
Table 3
| Effects | Post-mastectomy reconstruction | Implant-based reconstruction | Autologous reconstruction† | Contralateral prophylactic mastectomy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |||||
| Age, increasing age | 0.99 | 0.98, 0.99 | 0.002 | 1 | 0.99, 1.01 | 0.806 | 1.02 | 1.01, 1.03 | <0.001 | 0.99 | 0.98, 0.99 | 0.004 | ||||
| Year, more recent year | 1.06 | 1.04, 1.09 | <0.001 | 0.99 | 0.95, 1.02 | 0.446 | 0.9 | 0.87, 0.93 | <0.001 | 0.97 | 0.95, 0.99 | 0.003 | ||||
| Race | ||||||||||||||||
| Non-Hispanic White | Reference | Reference | Reference | Reference | ||||||||||||
| Non-Hispanic Black | 0.9 | 0.80, 1.00 | 0.06 | 0.81 | 0.71, 0.93 | 0.003 | 1.33 | 1.16, 1.51 | <0.001 | 0.65 | 0.60, 0.71 | <0.001 | ||||
| Hispanic | 0.88 | 0.77, 1.02 | 0.085 | 1 | 0.87, 1.15 | 1 | 0.97 | 0.82, 1.15 | 0.716 | 0.61 | 0.55, 0.68 | <0.001 | ||||
| Other | 0.79 | 0.69, 0.91 | 0.001 | 0.98 | 0.82, 1.17 | 0.834 | 1.01 | 0.86, 1.19 | 0.866 | 0.54 | 0.48, 0.61 | <0.001 | ||||
| Charlson-Deyo co-morbidity score | ||||||||||||||||
| 0 | Reference | Reference | Reference | Reference | ||||||||||||
| ≥1 | 1 | 0.87, 1.15 | 0.987 | 1.01 | 0.86, 1.17 | 0.927 | 1.16 | 1.00, 1.34 | 0.052 | 1.17 | 1.04, 1.33 | 0.007 | ||||
| Insurance | ||||||||||||||||
| Private | Reference | Reference | Reference | Reference | ||||||||||||
| Uninsured | 0.35 | 0.28, 0.44 | <0.001 | 0.99 | 0.75, 1.33 | 0.987 | 1.09 | 0.81, 1.48 | 0.565 | 0.66 | 0.54, 0.81 | <0.001 | ||||
| Public | 0.55 | 0.50, 0.61 | <0.001 | 0.98 | 0.88, 1.10 | 0.733 | 1.01 | 0.89, 1.14 | 0.91 | 0.83 | 0.77, 0.90 | <0.001 | ||||
| Income | ||||||||||||||||
| ≤$50,353 | Reference | Reference | Reference | Reference | ||||||||||||
| ≥$50,354 | 1.54 | 1.38, 1.71 | <0.001 | 0.97 | 0.84, 1.10 | 0.608 | 1.03 | 0.90, 1.18 | 0.626 | 0.9 | 0.83, 0.98 | 0.017 | ||||
| Education level | ||||||||||||||||
| Low | Reference | Reference | Reference | Reference | ||||||||||||
| High | 1.07 | 0.98, 1.17 | 0.145 | 1.16 | 1.04, 1.29 | 0.007 | 0.84 | 0.76, 0.94 | 0.002 | 1.13 | 1.05, 1.23 | 0.003 | ||||
| Distance from facility | ||||||||||||||||
| <10 miles | Reference | Reference | Reference | Reference | ||||||||||||
| 11–50 miles | 0.93 | 0.85, 1.01 | 0.076 | 1.06 | 0.98, 1.16 | 0.14 | 0.98 | 0.89, 1.08 | 0.641 | 1.13 | 1.06, 1.21 | <0.001 | ||||
| >50 miles | 1.01 | 0.84, 1.21 | 0.917 | 0.96 | 0.79, 1.17 | 0.678 | 1.01 | 0.83, 1.24 | 0.918 | 0.93 | 0.83, 1.05 | 0.245 | ||||
| HR status | ||||||||||||||||
| HR+/HER2− | Reference | Reference | Reference | Reference | ||||||||||||
| HR+/HER2+ | 0.86 | 0.76, 0.97 | 0.017 | 0.95 | 0.83, 1.09 | 0.465 | 1.07 | 0.92, 1.24 | 0.37 | 1.08 | 0.97, 1.21 | 0.148 | ||||
| HR−/HER2+ | 0.9 | 0.80, 1.02 | 0.094 | 0.96 | 0.83, 1.10 | 0.566 | 1.07 | 0.93, 1.24 | 0.341 | 0.97 | 0.87, 1.09 | 0.676 | ||||
| HR−/HER2− | 0.79 | 0.72, 0.87 | <0.001 | 0.94 | 0.85, 1.04 | 0.221 | 1.07 | 0.97, 1.19 | 0.178 | 1.26 | 1.16, 1.38 | <0.001 | ||||
| Grade | ||||||||||||||||
| I–II | Reference | Reference | Reference | Reference | ||||||||||||
| III–IV | 0.88 | 0.82, 0.95 | 0.001 | 1 | 0.92, 1.09 | 0.951 | 0.96 | 0.87 | 1.05 | 0.361 | 1.04 | 0.97, 1.11 | 0.256 | |||
| Clinical T stage | ||||||||||||||||
| 1–2 | Reference | Reference | Reference | Reference | ||||||||||||
| 3–4 | 1.6 | 1.47, 1.74 | <0.001 | 1.06 | 0.95, 1.19 | 0.27 | 1.04 | 0.91, 1.20 | 0.524 | 0.78 | 0.72, 0.84 | <0.001 | ||||
| Clinical N stage | ||||||||||||||||
| 0 | Reference | Reference | Reference | Reference | ||||||||||||
| 1–3 | 0.75 | 0.69, 0.82 | <0.001 | 1.03 | 0.93, 1.14 | 0.515 | 1.02 | 0.91, 1.15 | 0.692 | 0.99 | 0.92, 1.06 | 0.797 | ||||
| Receipt of chemotherapy | ||||||||||||||||
| None | Reference | Reference | Reference | Reference | ||||||||||||
| Neoadjuvant chemotherapy | 1.04 | 0.91, 1.18 | 0.597 | 0.93 | 0.82, 1.62 | 0.297 | 1.04 | 0.91, 1.19 | 0.542 | 1.34 | 1.21, 1.50 | <0.001 | ||||
| Adjuvant chemotherapy | 0.87 | 0.78, 0.96 | 0.009 | 1.01 | 0.89, 1.14 | 0.905 | 1.05 | 0.93, 1.18 | 0.441 | 1.17 | 1.07, 1.28 | 0.001 | ||||
| Receipt of radiation | ||||||||||||||||
| None | Reference | Reference | Reference | Reference | ||||||||||||
| Radiation | 0.75 | 0.69, 0.82 | <0.001 | 0.98 | 0.89, 1.08 | 0.783 | 1.03 | 0.93, 1.14 | 0.579 | 0.9 | 0.84, 0.97 | 0.005 | ||||
| Axillary surgery | ||||||||||||||||
| Sentinel lymph node biopsy | Reference | Reference | Reference | Reference | ||||||||||||
| No surgery | 0.61 | 0.47, 0.79 | <0.001 | 0.7 | 0.49, 1.00 | 0.051 | 1.19 | 0.74, 1.59 | 0.67 | 0.99 | 0.75, 1.29 | 0.925 | ||||
| Axillary lymph node dissection | 0.68 | 0.63, 0.74 | <0.001 | 1.03 | 0.94, 1.13 | 0.5 | 1.16 | 1.05, 1.27 | 0.002 | 0.91 | 0.84, 0.98 | 0.013 | ||||
| Mastectomy type | ||||||||||||||||
| Unilateral mastectomy | Reference | N/A | N/A | N/A | ||||||||||||
| Contralateral prophylactic mastectomy | 1.27 | 1.16, 1.39 | <0.001 | |||||||||||||
| Receipt of reconstruction | ||||||||||||||||
| No | N/A | N/A | N/A | Reference | ||||||||||||
| Yes | 1.27 | 1.16, 1.40 | <0.001 | |||||||||||||
†, patients with “autologous and implant” reconstruction were included in the autologous group. NCDB, National Cancer Database; OR, odds ratio; CI, confidence interval; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; N/A, not available.
Post-mastectomy reconstruction type
In the MR group, implant-based reconstruction was most common (37.3%), followed by autologous (26.1%), and combined-type reconstruction (10.1%). When stratified by surgery type, implant-based reconstruction was most common after all mastectomy types and made up nearly half of the reconstruction cases for patients who underwent CPM (44.5%) (Figure 2). Autologous reconstruction was the second most common type of reconstruction across groups. Since patients who completed NSM are coded in the NCDB as having “unknown” type of reconstruction, the “unknown” group accounts for NSM as well as a small number in whom reconstruction type is truly unaccounted for.
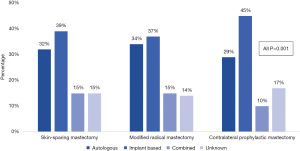
Factors associated with post-mastectomy reconstruction type
After controlling for patient (age, year, race, Charlson-Deyo score, insurance, income, education), facility (distance), tumor (HR status, grade, clinical T and N stage), and treatment characteristics (radiation, chemotherapy, axillary surgery, and mastectomy type), the only factor positively associated with implant-based reconstruction was higher level of education (OR 1.16, P=0.007) (Table 3). Factors positively associated with autologous reconstruction included increasing age (OR 1.02, P<0.001), non-Hispanic Black race (OR 1.33, P<0.001), and ALND (1.16, P=0.002). Patients treated in more recent years (OR 0.90, P<0.001) and those with higher education (OR 0.84, P=0.002) were less likely to undergo autologous reconstruction.
Factors associated with CPM
Patients with TNBC were nearly 30% more likely to undergo CPM (OR 1.26, P<0.001) than those with HR+/HER2− disease; MR patients were also 30% more likely to have CPM (OR 1.27 P<0.001) (Table 3). Compared to patients not treated with chemotherapy, those who received NAC or adjuvant chemotherapy were more likely to undergo CPM (OR 1.34, OR 1.17, respectively, both P≤0.001). Patients of non-White race and those with no insurance or public insurance were less likely to undergo CPM. More advanced cT stage (OR 0.78, P<0.001) and ALND (OR 0.91, P=0.013) were negatively associated with CPM (Table 3).
Factors associated with OS
On Kaplan-Meier analysis, 5-year OS was superior in the MR group (92% MR vs. 85% MA, P<0.001) (Figure 3A). When stratified by receipt of reconstruction and CPM, CPM did not confer a significant survival benefit in either MR or MA (Figure 3B). On multivariable Cox regression for all patients in the cohort, increasing age (hazard ratio 0.97, P<0.001) and Hispanic race (hazard ratio 0.80, P=0.011) were associated with superior OS (Table 4). Non-Hispanic Black race was associated with poorer OS as compared to non-Hispanic White race (hazard ratio 1.16, P=0.045), as was public insurance compared to private (hazard ratio 1.39, P<0.001). Compared to HR+/HER2− tumors, HR+/HER2+ and HR−/HER2+ tumors were associated with improved OS, but OS was not significantly worse in TNBC. More advanced T and N stage at diagnosis and higher tumor grade were associated with poorer OS, as were receipt of NAC (hazard ratio 1.47, P=0.003) and radiation (hazard ratio 1.13, P=0.044). Patients who received reconstruction (hazard ratio 0.76, P<0.001) and those who underwent CPM had improved survival (hazard ratio 0.80, P<0.001) compared to those who did not.
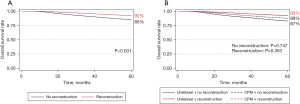
Table 4
| Effects | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age, increasing age | 0.97 | 0.96, 0.98 | <0.001 |
| Year, more recent year | 0.99 | 0.95, 1.02 | 0.395 |
| Race | |||
| Non-Hispanic White | Reference | ||
| Non-Hispanic Black | 1.16 | 1.01, 1.34 | 0.045 |
| Hispanic | 0.8 | 0.67, 0.95 | 0.011 |
| Other | 0.76 | 0.63, 0.92 | 0.004 |
| Charlson-Deyo co-morbidity score | |||
| 0 | Reference | ||
| ≥1 | 1.11 | 0.92, 1.34 | 0.264 |
| Insurance | |||
| Private | Reference | ||
| Uninsured | 1.16 | 0.89, 1.52 | 0.259 |
| Public | 1.39 | 1.23, 1.57 | <0.001 |
| Income | |||
| ≤$50,353 | Reference | ||
| ≥$50,354 | 0.95 | 0.85, 1.08 | 0.472 |
| Education level | |||
| Low | Reference | ||
| High | 1.01 | 0.89, 1.14 | 0.846 |
| Distance to facility | |||
| <10 miles | Reference | ||
| 11–50 miles | 1.08 | 0.98, 1.20 | 0.129 |
| >50 miles | 1.14 | 0.95, 1.36 | 0.156 |
| HR status | |||
| HR+/HER2− | Reference | ||
| HR+/HER2+ | 0.54 | 0.43, 0.68 | <0.001 |
| HR−/HER2+ | 0.45 | 0.36, 0.56 | <0.001 |
| HR−/HER2− | 1.18 | 0.98, 1.42 | 0.078 |
| Grade | |||
| I–II | Reference | ||
| III–IV | 1.78 | 1.56, 2.03 | <0.001 |
| Clinical T stage | |||
| 1–2 | Reference | ||
| 3–4 | 0.58 | 0.52, 0.64 | <0.001 |
| Clinical N stage | |||
| 0 | Reference | ||
| 1–3 | 1.63 | 1.42, 1.85 | <0.001 |
| Receipt of chemotherapy | |||
| No chemotherapy | Reference | ||
| Neoadjuvant chemotherapy | 1.47 | 1.14, 1.90 | 0.003 |
| Adjuvant chemotherapy | 1.09 | 0.85, 1.39 | 0.498 |
| Receipt of endocrine therapy | |||
| No | Reference | ||
| Yes | 0.54 | 0.45, 0.64 | <0.001 |
| Receipt of radiation | |||
| No radiation | Reference | ||
| Radiation | 1.13 | 1.00, 1.28 | 0.044 |
| Breast surgery | |||
| Unilateral mastectomy | Reference | ||
| Contralateral prophylactic mastectomy | 0.8 | 0.73, 0.88 | <0.001 |
| Axillary lymph node surgery | |||
| Sentinel lymph node biopsy | Reference | ||
| No axillary surgery | 1.03 | 0.68, 1.55 | 0.888 |
| Axillary lymph node dissection | 0.56 | 0.49, 0.63 | <0.001 |
| Receipt of reconstruction | |||
| No | Reference | ||
| Yes | 0.76 | 0.68, 0.84 | <0.001 |
NCDB, National Cancer Database; CI, confidence interval; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Therapy response in patients treated with NAC
Significantly more patients in the MR group achieved in-breast pCR (35.1% vs. 26.7% MA, P<0.001) (Figure S1). Similarly, axillary pCR (45.1% vs. 37.9%, P<0.001) and overall pCR (25.9% vs. 21.3%, P<0.001) were more common in MR than MA. On multivariable logistic regression, overall pCR (OR 1.20, P=0.021) and in-breast pCR (OR 1.30, P=0.003) were associated with MR, but not with CPM (Table S1). On multivariable Cox regression, those who achieved overall pCR, in-breast pCR, and axillary pCR (hazard ratio 0.20, 0.41, 0.34, all P<0.001) had superior OS (Table S2).
Discussion
Our study identifies factors associated with post-mastectomy reconstruction in a large, contemporary, nationally representative sample of young women with unilateral invasive breast cancer. We found that reconstruction was performed less often in minority groups and those with lower socioeconomic advantage, as well as for patients with more advanced disease and less favorable prognosis. Interestingly, in this cohort of patients age ≤40 years, CPM rates were universally high regardless of receipt of reconstruction, though CPM was independently associated with greater likelihood of reconstruction. Additionally, our data shows a survival benefit associated with both reconstruction and CPM—a finding which may be due to inherent selection bias and requires further investigation.
In our cohort, patients who received reconstruction were more often White, privately insured, and reported higher income levels compared to patients who did not undergo reconstruction. This is similar to findings from a 2000–2014 SEER database study of 321,206 women showing that MR was less likely in patients aged ≤40 years who were non-White (OR 0.68, 95% CI: 0.62–0.75), not married (0.83, 95% CI: 0.75–0.92), and located in the South or West regions of the United States (21). In studies not stratified by age, persistent disparities in race, income, education and insurance status affect receipt of reconstruction (20,25,26), as do geographic location, facility type, and access to plastic surgeons (26,27). In a 2004–2015 NCDB analysis of breast cancer patients ≥70 years old undergoing mastectomy, Cortina et al. demonstrated that patients who were White, had private insurance, and treated in the Northeast and in metropolitan areas were more likely to undergo reconstruction (22).
In our study, less favorable disease features and more comprehensive multimodal therapy were negatively associated with MR. Patients with TNBC (OR 0.79, P<0.001) and those with cN+ disease (OR 0.75, P<0.001) were less likely to undergo reconstruction. Others have also shown that higher AJCC stage, nodal positivity, larger tumor size, higher grade, and HR− disease decrease the likelihood of MR (19,20,22,25,28,29). In our cohort, patients with cT3–4 tumors were 60% more likely to undergo reconstruction than those with cT1–2 disease, but this may be due to the use of NAC and subsequent therapy response. In fact, while NAC was not independently associated with MR, those who achieved overall or breast pCR were 20% and 30% more likely to undergo reconstruction, respectively. Conversely, adjuvant chemotherapy was associated with a modest decrease in likelihood of MR (OR 0.87, P=0.009). This may be due to factors such as genomic testing results or more advanced disease at surgical pathology prompting adjuvant chemotherapy and radiation recommendations, patients being less inclined to undergo additional surgery unrelated to oncologic outcome, and desire to avoid delays in adjuvant treatment due to recovery time and potential complications associated with reconstruction (30). We also hypothesize that patients who receive NAC and have a favorable response may be more likely to elect reconstruction due to provider willingness to offer this procedure and time available for plastic surgery consultation and decision-making.
As expected, and consistent with others, our study found receipt of radiation was associated with a lower likelihood of MR (OR 0.75, P<0.001) (29,31). This may be related to radiation oncology preference for no reconstruction at the time of post-mastectomy radiation delivery and the increased incidence of complications and inferior cosmesis when radiation follows reconstruction (32-34).
In our contemporary cohort of young women, similar to earlier studies not stratified by age, implant-based reconstruction was the most common type (11,35,36), and in our study, this rate was particularly high in patients that underwent CPM. Unfortunately, the NCDB codes all reconstruction following NSM as “unknown”, thus our analysis cannot draw conclusions on reconstruction type for these patients. However, the desire for nipple preservation may be partially motivating high rates of reconstruction and CPM, as “unknown” accounts for over 12% of our MR cohort and 17% of the CPM group.
The majority of all patients in our study underwent CPM regardless of receipt of reconstruction (60.6% MR and 50.9% MA). While CPM rates are higher in younger women, and reach 35% in other series (37-39), our data may reflect more current trends since our cohort spanned 2010–2018. CPM was 30% more likely in patients who received NAC and 20% more likely in those treated with adjuvant chemotherapy, potentially reflecting patients’ desire for “peace of mind” after receiving a cancer diagnosis and treatment (12,40,41). Mirroring factors associated with reconstruction, ours and prior research demonstrates that patients are more likely to undergo CPM if they are younger, White, have private insurance, or less advanced disease (13,22,35,37,41). Additionally, we found a strong relationship between CPM and MR; each procedure was independently associated with a 30% increased likelihood of the other (both OR 1.27, P<0.001). Other studies have also shown a close association between reconstruction and bilateral mastectomy, particularly in younger women (11,26,35-37).
While information not available in the NCDB, such as genetic and family risk factors, likely contribute to the high rate of CPM in women ≤40 years, psychosocial factors and misunderstanding of breast cancer prognosis and contralateral breast cancer (CBC) risk also play an important role (11,13,42). In one retrospective study of 100 CPM patients at a single institution, 58% did not have a medical indication (i.e., pathogenic variant carrier) for the procedure (43). Instead, patients may be choosing CPM due to misplaced anxiety about breast cancer recurrence, preference to avoid future imaging surveillance, as well as concern about aesthetic outcomes and breast symmetry. This was evidenced in a mixed-methods study evaluating patients’ decisions for CPM and reconstruction, in which 82.4% of patients who chose CPM reported improved quality of life characterized by relief from worry about future cancer; CPM also yielded higher mean scores for satisfaction with breast (82.4% vs. 70.6%) and with outcome (98.9% vs. 75.2%) (44). Further, in a survey study of 3,631 women with newly diagnosed unilateral stage 0–II breast cancer, 37.3% of patients who received CPM believed it improved survival for all women with breast cancer (41). These findings suggest that new methods for patient education about the likelihood of CBC, potential risks of CPM, and lack of survival benefit are needed to help address these misconceptions. In addition, recent evidence suggesting a survival benefit with BCT compared to mastectomy, particularly for younger women (14-17) should be shared with patients who are appropriate candidates for either procedure from an oncologic and genetic standpoint, and might discourage the decision for unilateral mastectomy, which itself often prompts CPM.
Somewhat surprisingly, both receipt of CPM and MR were associated with an OS benefit on multivariable Cox regression in our study. This is in contrast to established research that consistently demonstrates no survival advantage for MR or CPM in patients without an increased risk of CBC (45-49). However, other retrospective analyses have shown contradictory results (50,51), including a survival benefit with CPM particularly in younger women with HR− breast cancer (52). Our findings likely reflect inherent cofounders in our analysis, or a selection bias towards performing CPM and MR in patients with a favorable prognosis. Since the NCDB does not include personal risk factors such as family history and genetic testing information, these variables are not accounted for in our study. Our OS benefit could also be explained by potential disparate populations of young breast cancer patients: one group undergoing early screening due to family history or pathogenic variants in whom breast cancers are detected at early stage, and another group who present with palpable breast tumors or clinically positive axilla. The treatment recommended to each group would differ, as would their oncologic outcomes, and perhaps MR and CPM are prioritized differently by both patients and providers in each group.
Like other studies based on large retrospective datasets, ours has limitations. Local-regional recurrence and disease-specific survival are not included in the NCDB, limiting our outcomes to OS, an imperfect measure especially in a cohort of young women. The NCDB does not collect data on smoking status or body mass index (BMI), which are important potential contraindications to immediate reconstruction, and/or additional surgery such as CPM. Facility type and location variables are suppressed for patients <40 years due to low sample size, preventing comparison to other studies examining these variables. Since the NCDB does not provide information on timing of reconstruction, there is no way to determine whether it was truly immediate, delayed, or intermediate/delayed (i.e., immediate tissue expander placement, delayed autologous reconstruction), all of which are quite different in clinical practice. The inability to differentiate which patients received immediate vs. delayed reconstruction could have biased our OS results, assuming that delayed reconstruction was necessary due to advanced disease requiring adjuvant chemotherapy and/or radiation and that this group had inferior prognosis compared with patients who received immediate reconstruction. However, there are likely fewer differences between the two groups who received reconstruction (immediate vs. delayed) than the groups that received any reconstruction vs. no reconstruction since non-oncologic surgery is generally only offered when disease is limited or has responded well to systemic therapy and/or radiation, prognosis is sufficiently favorable, and the patient’s overall health is amenable to additional procedures and recovery. Additionally, there are no data in the NCDB on surgical decision-making factors such as aesthetic concerns and genetic or family risk of breast cancer, which likely contribute to the high CPM rates in young women. We selected the 2010–2018 cohort to ensure complete prognostic marker data since HER2 status was collected beginning in 2010, and to contribute a modern study to the literature on post-mastectomy reconstruction and CPM. While using this more contemporary cohort limited our sample size, our analysis is one of the largest and most nationally representative to address these questions in the unique group of young breast cancer patients.
Conclusions
In summary, our study shows persistent disparities amongst women age ≤40 years with unilateral invasive breast cancer in receipt of MR and CPM. Despite the Women’s Health and Cancer Rights Act of 1998’s provision of financial protection to women who choose to undergo mastectomy and reconstruction, our data reflecting cancer care 20 years later suggest that lack of private insurance and lower income may still be potential barriers (53). While more advanced disease and receipt of multimodal treatment may discourage patients from pursuing non-essential procedures such as reconstruction and CPM, breast surgeons should ensure access and equitable care are available to all women with all types of breast cancer. We must also ensure our patients are adequately educated about the true risks and benefits of post-mastectomy reconstruction and CPM to empower them to make well-informed decisions.
Acknowledgments
This study was presented as an oral presentation at the 18th Annual Academic Surgical Congress, February 7−9, 2023, Houston, TX, USA.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-23-48/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-23-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-23-48/coif). A.S. receives honoraria as a speaker for Endomag. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board exemption was obtained as the data source is deidentified and this was a retrospective cohort study. Inform consent was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin 2022;72:524-41. [Crossref] [PubMed]
- Hironaka-Mitsuhashi A, Tsuda H, Yoshida M, et al. Invasive breast cancers in adolescent and young adult women show more aggressive immunohistochemical and clinical features than those in women aged 40-44 years. Breast Cancer 2019;26:386-96. [Crossref] [PubMed]
- Hu X, Myers KS, Oluyemi ET, et al. Presentation and characteristics of breast cancer in young women under age 40. Breast Cancer Res Treat 2021;186:209-17. [Crossref] [PubMed]
- Gnerlich JL, Deshpande AD, Jeffe DB, et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg 2009;208:341-7. [Crossref] [PubMed]
- Keegan TH, DeRouen MC, Press DJ, et al. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res 2012;14:R55. [Crossref] [PubMed]
- Rosenberg SM, Partridge AH. Management of breast cancer in very young women. Breast 2015;24:S154-8. [Crossref] [PubMed]
- Azim HA Jr, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res 2014;16:427. [Crossref] [PubMed]
- Azim HA Jr, Michiels S, Bedard PL, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res 2012;18:1341-51. [Crossref] [PubMed]
- Manrique OJ, Banuelos J, Abu-Ghname A, et al. Surgical Outcomes of Prepectoral Versus Subpectoral Implant-based Breast Reconstruction in Young Women. Plast Reconstr Surg Glob Open 2019;7:e2119. [Crossref] [PubMed]
- Sun ZH, Chen C, Kuang XW, et al. Breast surgery for young women with early-stage breast cancer: Mastectomy or breast-conserving therapy? Medicine (Baltimore) 2021;100:e25880. [Crossref] [PubMed]
- Agarwal S, Kidwell KM, Kraft CT, et al. Defining the relationship between patient decisions to undergo breast reconstruction and contralateral prophylactic mastectomy. Plast Reconstr Surg 2015;135:661-70. [Crossref] [PubMed]
- Dominici L, Hu J, Zheng Y, et al. Association of Local Therapy With Quality-of-Life Outcomes in Young Women With Breast Cancer. JAMA Surg 2021;156:e213758. [Crossref] [PubMed]
- Nash R, Goodman M, Lin CC, et al. State Variation in the Receipt of a Contralateral Prophylactic Mastectomy Among Women Who Received a Diagnosis of Invasive Unilateral Early-Stage Breast Cancer in the United States, 2004-2012. JAMA Surg 2017;152:648-57. [Crossref] [PubMed]
- Hartmann-Johnsen OJ, Kåresen R, Schlichting E, et al. Better survival after breast-conserving therapy compared to mastectomy when axillary node status is positive in early-stage breast cancer: a registry-based follow-up study of 6387 Norwegian women participating in screening, primarily operated between 1998 and 2009. World J Surg Oncol 2017;15:118. [Crossref] [PubMed]
- Li P, Li L, Xiu B, et al. The Prognoses of Young Women With Breast Cancer (≤35 years) With Different Surgical Options: A Propensity Score Matching Retrospective Cohort Study. Front Oncol 2022;12:795023. [Crossref] [PubMed]
- De la Cruz Ku G, Karamchandani M, Chambergo-Michilot D, et al. Does Breast-Conserving Surgery with Radiotherapy have a Better Survival than Mastectomy? A Meta-Analysis of More than 1,500,000 Patients. Ann Surg Oncol 2022;29:6163-88. [Crossref] [PubMed]
- Xiang W, Wu C, Wu H, et al. Survival Comparisons between Breast Conservation Surgery and Mastectomy Followed by Postoperative Radiotherapy in Stage I-III Breast Cancer Patients: Analysis of the Surveillance, Epidemiology, and End Results (Seer) Program Database. Curr Oncol 2022;29:5731-47. [Crossref] [PubMed]
- Kurian AW, Lichtensztajn DY, Keegan TH, et al. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998-2011. JAMA 2014;312:902-14. [Crossref] [PubMed]
- Gibreel WO, Day CN, Hoskin TL, et al. Mastectomy and Immediate Breast Reconstruction for Cancer in the Elderly: A National Cancer Data Base Study. J Am Coll Surg 2017;224:895-905. [Crossref] [PubMed]
- Butler PD, Familusi O, Serletti JM, et al. Influence of race, insurance status, and geographic access to plastic surgeons on immediate breast reconstruction rates. Am J Surg 2018;215:987-94. [Crossref] [PubMed]
- Nayyar A, Strassle PD, Reddy KG, et al. Variations in the utilization of immediate post-mastectomy breast reconstruction. Am J Surg 2019;218:712-5. [Crossref] [PubMed]
- Cortina CS, Bergom CR, Kijack J, et al. Postmastectomy breast reconstruction in women aged 70 and older: An analysis of the National Cancer Database (NCDB). Surgery 2021;170:30-8. [Crossref] [PubMed]
- Sada A, Day CN, Hoskin TL, et al. Mastectomy and immediate breast reconstruction in the elderly: Trends and outcomes. Surgery 2019;166:709-14. [Crossref] [PubMed]
- Partridge AH, Pagani O, Abulkhair O, et al. First international consensus guidelines for breast cancer in young women (BCY1). Breast 2014;23:209-20. [Crossref] [PubMed]
- Karadsheh MJ, Katsnelson JY, Ruth KJ, et al. Breast Reconstruction in Inflammatory Breast Cancer: An Analysis of Predictors, Trends, and Survival from the National Cancer Database. Plast Reconstr Surg Glob Open 2021;9:e3528. [Crossref] [PubMed]
- Mandelbaum A, Nakhla M, Seo YJ, et al. National trends and predictors of mastectomy with immediate breast reconstruction. Am J Surg 2021;222:773-9. [Crossref] [PubMed]
- Hershman DL, Richards CA, Kalinsky K, et al. Influence of health insurance, hospital factors and physician volume on receipt of immediate post-mastectomy reconstruction in women with invasive and non-invasive breast cancer. Breast Cancer Res Treat 2012;136:535-45. [Crossref] [PubMed]
- Nair AG, Giannakeas V, Semple JL, et al. Contemporary Trends in Breast Reconstruction Use and Impact on Survival Among Women with Inflammatory Breast Cancer. Ann Surg Oncol 2022;29:8072-82. [Crossref] [PubMed]
- van Egdom LSE, de Ligt KM, de Munck L, et al. Predictors of delayed breast reconstruction in the Netherlands: a 5-year follow-up study in stage I-III breast cancer patients. Breast Cancer 2022;29:324-35. [Crossref] [PubMed]
- Grigor EJM, Stein MJ, Arnaout A, et al. The effect of immediate breast reconstruction on adjuvant therapy delay, locoregional recurrence, and disease-free survival. Breast J 2021;27:857-62. [Crossref] [PubMed]
- Roder D, Zorbas H, Kollias J, et al. Factors predictive of immediate breast reconstruction following mastectomy for invasive breast cancer in Australia. Breast 2013;22:1220-5. [Crossref] [PubMed]
- Ho AY, Hu ZI, Mehrara BJ, et al. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol 2017;18:e742-53. [Crossref] [PubMed]
- Ho AL, Bovill ES, Macadam SA, et al. Postmastectomy radiation therapy after immediate two-stage tissue expander/implant breast reconstruction: a University of British Columbia perspective. Plast Reconstr Surg 2014;134:1e-10e. [Crossref] [PubMed]
- Casella D, Di Taranto G, Onesti MG, et al. A retrospective comparative analysis of risk factors and outcomes in direct-to-implant and two-stages prepectoral breast reconstruction: BMI and radiotherapy as new selection criteria of patients. Eur J Surg Oncol 2019;45:1357-63. [Crossref] [PubMed]
- Bekeny JC, Schreeder CA, Wirth P, et al. Factors contributing to persistent rates of contralateral prophylactic mastectomy in breast cancer patients: Examination of 1051 mastectomies across a single health system. Breast J 2020;26:2341-9. [Crossref] [PubMed]
- Albornoz CR, Matros E, Lee CN, et al. Bilateral Mastectomy versus Breast-Conserving Surgery for Early-Stage Breast Cancer: The Role of Breast Reconstruction. Plast Reconstr Surg 2015;135:1518-26. [Crossref] [PubMed]
- King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol 2011;29:2158-64. [Crossref] [PubMed]
- Tuttle TM, Habermann EB, Grund EH, et al. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203-9. [Crossref] [PubMed]
- Lazow SP, Riba L, Alapati A, et al. Comparison of breast-conserving therapy vs mastectomy in women under age 40: National trends and potential survival implications. Breast J 2019;25:578-84. [Crossref] [PubMed]
- Huang J, Chagpar A. Factors associated with decision to undergo contralateral prophylactic mastectomy versus unilateral mastectomy. Am J Surg 2019;218:170-4. [Crossref] [PubMed]
- Jagsi R, Hawley ST, Griffith KA, et al. Contralateral Prophylactic Mastectomy Decisions in a Population-Based Sample of Patients With Early-Stage Breast Cancer. JAMA Surg 2017;152:274-82. [Crossref] [PubMed]
- Montagna G, Morrow M. Contralateral prophylactic mastectomy in breast cancer: what to discuss with patients. Expert Rev Anticancer Ther 2020;20:159-66. [Crossref] [PubMed]
- Fairbairn K, Cervantes A, Rayhrer C, et al. Trends in Contralateral Prophylactic Mastectomy. Aesthetic Plast Surg 2020;44:323-9. [Crossref] [PubMed]
- Buchanan PJ, Abdulghani M, Waljee JF, et al. An Analysis of the Decisions Made for Contralateral Prophylactic Mastectomy and Breast Reconstruction. Plast Reconstr Surg 2016;138:29-40. [Crossref] [PubMed]
- Pesce C, Liederbach E, Wang C, et al. Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor-negative breast cancer. Ann Surg Oncol 2014;21:3231-9. [Crossref] [PubMed]
- Boughey JC, Attai DJ, Chen SL, et al. Contralateral Prophylactic Mastectomy (CPM) Consensus Statement from the American Society of Breast Surgeons: Data on CPM Outcomes and Risks. Ann Surg Oncol 2016;23:3100-5. [Crossref] [PubMed]
- Xiong M, Liu Z, Lv W, et al. Breast Reconstruction Does Not Affect the Survival of Patients with Breast Cancer Located in the Central and Nipple Portion: A Surveillance, Epidemiology, and End Results Database Analysis. Front Surg 2022;9:855999. [Crossref] [PubMed]
- Gradishar WJ, Lurie RH, Blair SL, et al. Invasive Breast Cancer NCCN Guidelines® NCCN Breast Cancer Panel Members. Vol 14.; 2016. Available online: http://www.cap.org.
- Carbine NE, Lostumbo L, Wallace J, et al. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev 2018;4:CD002748. [Crossref] [PubMed]
- Wong SM, Freedman RA, Sagara Y, et al. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg 2017;265:581-9. [Crossref] [PubMed]
- Herrinton LJ, Barlow WE, Yu O, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol 2005;23:4275-86. [Crossref] [PubMed]
- Bedrosian I, Hu CY, Chang GJ. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst 2010;102:401-9. [Crossref] [PubMed]
- Women’s Health and Cancer Rights Act. Cancer.Org. Published May 13, 2019. Accessed February 12, 2023. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/6037.00.pdf
Cite this article as: Silsby ZO, Drapalik LM, Amin AL, Simpson A, Rock L, Shenk R, Miller ME. Factors influencing post-mastectomy reconstruction in breast cancer patients aged 40 years and younger. Ann Breast Surg 2024;8:15.

