
Indocyanine green angiography for autologous breast reconstruction: a prospective observational study
Highlight box
Key findings
• Though use of intraoperative indocyanine green angiography (ICG-A) did not show a significant correlation with postoperative complications in autologous breast reconstructive procedures using pedicled latissimus dorsi (pLD)- and deep inferior epigastric perforator (DIEP)-flaps, an inadequate ICG-A may indicate compromised tissue perfusion prompting immediate intraoperative intervention.
What is known and what is new?
• Inadequate intraoperative ICG-A may indicate compromised tissue perfusion.
• In pLD-flap breast reconstructions, 52.6% of flaps demonstrated insufficient perfusion on ICG-A. Among the flaps with insufficient perfusion, 70% experienced postoperative complications, with 28.6% classified as Clavien-Dindo (CD) >3 complications.
• In DIEP-flap breast reconstructions, 68% showed insufficient perfusion on ICG-A. Among the flaps with insufficient perfusion, 64.7% experienced postoperative complications, with 63.6% classified as CD >3 complications.
• This study demonstrated the feasibility, application and utilization of intraoperative ICG-A in a broader setting involving multiple surgeons.
What is the implication, and what should change now?
• Future research including larger-scale studies are needed to obtain higher-quality data and more definitive conclusions.
Introduction
In 2020, 2.3 million women were diagnosed with breast cancer (1) affecting 1 in every 9 women (2), making breast cancer the leading cancer in women worldwide. The global breast cancer burden is estimated to raise with 47% from 2020 to 2040 (3). During the past two decades breast cancer screening has been introduced and treatment has become increasingly more effective. Though recent studies have indicated that breast conserving therapy may be the recommended treatment of early breast cancer (4,5), the demand for breast reconstruction after mastectomy remain (3,6).
In 1906, Tansini described the latissimus dorsi (LD)-flap for breast reconstruction (7). LD-flap anatomy was investigated by Schneider et al. in 1977 (8), and further developed by Bostwick et al. (9) using a skin island over the muscle. Papp and McCraw (10) developed the deepithelialized LD-flap for volume replacement in the start 1980’ies paving the way for today’s LD-design and surgical techniques.
Since Halsted (11) defined the gold standard for breast cancer surgery in 1889, autologous breast reconstruction (ABR) described in 1979 (12), has evolved as a safe and viable option encompassing among others the deep inferior epigastric perforator flap (DIEP-flap) and the pedicled latissimus dorsi flap (pLD-flap) (13).
Koshima et al. revolutionized breast reconstructive surgery in 1989 introducing the perforator-based DIEP-flap (14), used for ABR by Allen and Treece 1994 (15).
The DIEP-flap is characterized by a low donor site morbidity and an acceptable aesthetic result (16,17).
Since then, both the LD- and the DIEP-flap have become workhorses and is the more commonly used flaps for ABR (13,18-21).
The goal of breast reconstructive surgery is to achieve successful breast reconstruction, ensuring sufficient tissue perfusion, minimizing donor-site morbidity, and maximizing both clinical outcomes and aesthetic results.
Establishing a robust blood supply is of utmost importance during ABR to minimize potential complications, including partial or complete flap loss, fat necrosis (FN), and the need for additional surgical interventions (21).
Traditionally, assessment of flap perfusion is performed during surgery using clinical assessment, temperature, color, capillary refill, turgor and bleeding. However, all of the above depends on the clinical experience of the surgical team and may miss signs of insufficient perfusion (22,23).
Optimization of ABR implementing techniques able to identify tissue perfusion and ischemia has a potential to greatly benefit the patients and consequently society.
Indocyanine green angiography (ICG-A) was introduced in plastic surgery more than two decades ago and provides real-time intraoperative assessment of tissue perfusion (24). The use of ICG-A for breast reconstructive procedures has been reported to be associated with a lower risk of per- and postoperative complications (19,25-27), making this modality a valuable intraoperative assessment tool for the breast reconstructive surgeon (28).
Hypothesis
The utilization of peroperative ICG-A has the potential to enhance autologous breast reconstructive procedures by selecting relevant perforators and evaluating the degree of perfusion within the reconstructive flap. This approach is expected to decrease the occurrence of postoperative complications, including flap failure requiring surgical intervention, necrosis, hematoma, and infection.
Accordingly, we conducted a prospective observational study applying ICG-A in autologous breast reconstructive procedures using pLD-flaps and DIEP-flaps. The aim of this study was to visualize the peroperative tissue perfusion and correlate peroperative perfusion with postoperative complications. We present this article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-23-53/rc).
Methods
Patients
This prospective study included 41 patients undergoing immediate or delayed breast reconstruction by autologous perforator flaps, comprising a total of 25 DIEP-flaps and 21 pLD-flaps [16 full pLD-flaps and 5 muscle sparing latissimus dorsi (msLD)-flaps].
Patients were included consecutively from February 2020 to May 2021 at the Department of Plastic Surgery and Burns Treatment, Copenhagen University Hospital.
The inclusion criteria were: patients older than 18 years of age deemed suitable for ABR by the consultant plastic and breast surgeon. Patients who were pregnant, breastfeeding or not able to understand enough Danish to comprehend the given information and to complete the study questionnaires were excluded. Patients allergic to iodine were also excluded (29).
Data collection
Authors adhered to STROBE guidelines. All data is obtained and stored anonymously [according to the General Data Protection Regulation (GDPR)] by the corresponding author (E.L.).
Data from the electronic patient files (Sundhedsplatformen, Epic Systems Corporation®, Verona, WI, USA) and peroperative data was obtained and stored anonymously according to regulations by The Danish Data Protection Agency (PACTIUS).
Ethical considerations
The study was approved by the Capital Denmark Region Committees on Health Research Ethics (H-19074897, 70432) and conducted in accordance with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the patients.
ICG-A assessment
Indocyanine green (ICG) is a water-soluble fluorescent molecule that emits fluorescence when illuminated with a near infrared light using the SPY-Elite Fluorescence Imaging system® (Stryker AB, Malmö, Sweden, www.stryker.com). The procedure can be repeated multiple times during surgery due to a short half-life of ICG (30).
Approximately 20 seconds upon intravenous injection of 2.5 mL/mg Verdye® (Diagnostic Green GmbH Feldkirchener Str. 7c 85551 Kirchheim b. Munich, Germany), followed by 10 mL saline flush, peroperative tissue perfusion was visualized Figure 1. Perfusion values were scored and quantified after 45 seconds, and the recording terminated after 60 seconds (31). Perfusion assessment and quantification of relative values were performed by the same investigator, with a minimum of 20 minutes between each ICG injection (30).
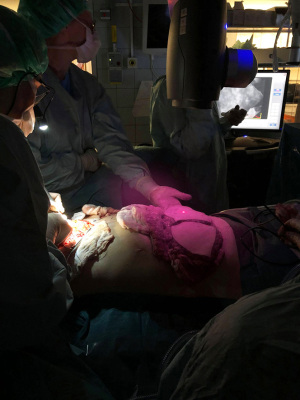
The quantification of peroperative ICG-A values were done by measuring perfusion in the circumference and across the surface of the entire flap. Healthy tissue outside the surgical field was used as reference point (100%).
A relative cut-off value was set at 33% of perfusion in healthy tissue (31), with values <33% leading to reevaluation of the procedure. Reevaluation included comparison of the clinical assessment to the angiography in order to assess areas with poor perfusion and perform resection, is necessary.
Peroperative ICG-A application and perfusion assessment
pLD flap
Preoperatively, the flap is marked while the patient is in a standing position, marking the skin paddle overlying the LD muscle and ultrasonography is performed to identify and mark the perforators or artery(ies) on the skin island.
Peroperatively, ICG-A was applied 3 times at three preset timepoints assessing perfusion. Each angiography was compared to the surgeon’s clinical assessment of perfusion (temperature, color, capillary refill, turgor and bleeding).
The first angiography was done after incision to the fascia, indicating the number of perforators within the flap.
ICG-A was then repeated after the flap was raised on its pedicle—before transposition/advancement, allowing assessment of the chosen perforator or artery to evaluate possible changes in perfusion—assessing the angiosome (if the flap was designed as a perforator flap) (Figure 2A,2B).
The final ICG-A was performed after the flap was transposed to the recipient site (the breast) and the breast reconstruction finalized.
In case of clinical and/or suspected insufficient perfusion by the ICG-A, assessment of perfusion was reevaluated (clinical- and ICG-A reevaluation). Upon reevaluation resection of tissue and/or change of intraoperative surgical strategy was done accordingly, and ICG-A repeated Videos 1,2.
DIEP flap
Preoperative computed tomography angiography (CTA) is gold standard for surgical planning including perforator mapping and selection (32).
Preoperatively, CTA was performed in all cases to identify the dominant perforator and the intramuscular course of the vessels in the flap. Peroperative handheld doppler ultrasonography confirming the localized perforators identified by the CTA was performed right after inducing general anesthesia, before initiating surgery.
ICG-A was performed at five predetermined intervals during surgery comparing each angiography to the surgeon’s clinical assessment of perfusion (Figure 3).

The initial angiography was done upon incision around the flap to the fascial level—before entering the subfascial plane—to identify the complete number of perforators entering the flap. This ICG-A was then compared to the preoperative CTA. Based on this comparison, the best and most reliable perforator(s) were dissected. The second ICG-A was done after the selected perforators were dissected but before incision of the rectus abdominis fascia.
A third ICG-A was done after the flap was raised with complete pedicle dissection allowing a final assessment of flap perfusion before transposition to the breast. Perfusion was evaluated by a fourth angiography upon transposition to the recipient site and completing the microvascular anastomoses (Figure 2A,2B). The fifth and final ICG-A was performed after reconstruction was completed. By repeating angiographies at these five preset timepoints, surgeons were able to evaluate and reevaluate perfusion multiple times to identify possible insufficiently perfused areas of the flap, allowing for optimal perfusion assessment supporting the intraoperative decision-making Videos 3,4. In case of insufficient perfusion (Figure 4A,4B), clinical reevaluation adjusting the reconstructive procedure was done and the angiography repeated (Video 5).
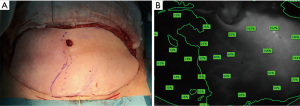
Individual charts for each patient noting the dominant perforator(s), clinical assessments, ICG-A findings and flap weight before and after trimming were done intraoperatively.
Complications
The Clavien-Dindo (CD) classification system was utilized to categorize postoperative complications (33). If a patient developed complications that fell into different CD grades, they were classified according to the highest grade.
Follow-up and postoperative clinical evaluation
Clinical evaluations were performed 4 times upon trial inclusion: before surgery and 3 times during the follow-up period (4 weeks, 4–6 months and 12 months postoperatively). Outpatient visits encompassed an overall assessment of patient well-being and clinical examination including inspection of the surgical fields (signs of infection, necrosis, seroma, hematoma, etc.), scar assessment by the Patient and Observer Scar Assessment Score (POSAS), BREAST-Q questionnaires and lymphedema measurements. Furthermore, participating patients attended the same standard post-operative follow-up in the outpatient clinic as non-participating patients undergoing ABR. Clinical follow-up was completed for all patients by May 2022.
Patient reported satisfaction and aesthetic outcome
Patients completed the Danish version of the BREAST-Q 1.0 pre-reconstruction module before surgery (at baseline), and the BREAST-Q 1.0 post-reconstruction module at each clinical follow-up (34). Quality of life (QoL) was evaluated using the BREAST-Q score.
POSAS 2.0
Scars were evaluated by both the patient and the clinician at each post-operative clinical visit using the POSAS (35).
Assessment of postoperative lymphedema
Consecutive clinical evaluation and measurements of the affected limb/limbs was done to assess the incidence of postoperative lymphedema, by measurements before surgery (baseline) and at each postoperative visit (36).
A specialized physiotherapist performed the lymphedema assessment. Assessments included physical examination, circumferential measurements (figure-of-eight) and by bioimpedance spectroscopy (BIS), the system called SOZO (https://www.impedimed.com/products/sozo/).
Presence of lymphedema was categorized according to the International Society of Lymphology staging system (37).
Endpoints
Our primary endpoint was to analyze the proportion of flaps where the ICG-A assessment showed sufficient/insufficient perfusion, and the proportion where the peroperative ICG-A had a clinical implication for the surgical decision making. Finally, association between intraoperative ICG-A and per- and postoperative complications: infection, hematoma, necrosis, epidermolysis, partial- or full-flap loss and FN was evaluated (38).
In addition, for the DIEP-flap procedures, the association between the perforator identified preoperatively by the CTA and the perforators ultimately selected intraoperatively by the ICG-A was evaluated.
Statistical analysis
Statistical analysis was performed using R for Mac OS X GUI R 4.2.2 GUI 1.79 High Sierra build [8160]. R: Copyright © 2004–2021. The R Foundation for Statistical Computing (http://www.R-project.org).
Categorical variables were analyzed using Fisher’s exact test due to small sample size.
Outcome comparisons of the POSAS were made using Wilcox-signed rank test not assuming distributions are normal, to evaluate any differences between the patient’s and observer’s score.
BREAST-Q raw scores were transformed by the Qscore Software program version 1.6.3414.40306 licensed to Sloan-Kettering Institute for Cancer Research, developed by Rumm Laboratory. Statistical significance was set by P values <0.05. The BREAST-Q was analyzed comparing pre- and postoperative scores. Analyzing repetitive measurements using paired t-tests based on the assumption of normative data (39).
Results
Of 41 included patients, 5 dropped out leaving 36 patients for inclusion. Eighteen patients receiving a breast reconstruction using pLD-flaps and 18 patients receiving DIEP-flaps.
A total of 44 breast reconstructions were performed: 25 DIEP-flaps and 19 pLD-flaps (14 pLD-flaps and 5 msLD-flaps). Mean age was 50.5 years (range, 28–75 years) and mean body mass index (BMI) 23.5 kg/m2 (range, 17.9–32 kg/m2). Three patients were active smokers: 2 in the pLD-group and 1 in the DIEP-group, there were no significant difference in outcomes between smokers and non-smokers. Baseline demographics are depicted in Table 1. Table 2 depicts patient characteristics specified according to either pLD- or DIEP-flap-based breast reconstructions.
Table 1
| Characteristics | Data |
|---|---|
| Total No. of patients (n) | 41 (5 dropouts) |
| Patients included (n) | 36 |
| No. flaps (n) | |
| pLD | 19 |
| DIEP | 25 |
| Age (mean), years | 50.5 |
| BMI (mean), kg/m2 | 23.5 |
| Smoking status (n) | |
| Never | 27 |
| Former | 6 |
| Active | 3 |
| Alcohol (drinks per week) (mean) | 4.2 |
| Co-morbidities (n) | |
| Hypercholesterolemia | 2 |
| Hypertension | 3 |
| Psoriasis | 1 |
| IBD | 1 |
| Asthma | 4 |
| Hypothyreosis | 2 |
| Migraine | 1 |
| Menopause status (n) | |
| Premenopausal | 25 |
| Postmenopausal | 11 |
| Diagnosis/indication for surgery (n) | |
| C. Mamma | 31 |
| DCIS | 2 |
| Prophylactic surgery | 3 |
| Disposition (gene mutations) (n) | |
| BRCA 1 | 6 |
| BRCA 2 | 5 |
| CDH1-gene | 1 |
| Li Fraumeni mutation | 1 |
| Duration of surgery (mean), min | 341.8 |
| Mastectomy weight (mean), g | 418.2 |
| SN/axillary lymph node status (n) | |
| SNB | 13 |
| ALND | 20 |
| None | 3 |
| ICG-A dose (per-opr.), mg/mL | 2.5 (Verdye® 5) |
No., number; pLD, pedicled latissimus dorsi; DIEP, deep inferior epigastric perforator; BMI, body mass index; IBD, inflammatory bowel disease; C. Mamma, cancer mammae; DCIS, ductal carcinoma in situ; BRCA, breast cancer gene; CDH1, epithelial cadherin; SN, sentinel node; SNB, sentinel node biopsy; ALND, axillary lymph node dissection; ICG-A, indocyanine green angiography; per-opr., peroperative.
Table 2
| Characteristics according to type of flap | pLD-flaps | DIEP-flaps |
|---|---|---|
| Total No. of patients (n) | 18 | 18 |
| No. flaps (n), total | 19 | 25 |
| Unilateral | 17 | 11 |
| Bilateral | 1 | 7 |
| Timing of reconstruction (n), flaps | ||
| Immediate | 8 | 5 |
| Delayed | 11 | 20 |
| Age (mean), years | 50.1 | 51 |
| BMI (mean), kg/m2 | 22.2 | 24.8 |
| Alcohol (drinks per week) (mean) | 4.5 | 3.8 |
| Menopause status (n), patients | ||
| Premenopause | 12 | 13 |
| Postmenopause | 6 | 5 |
| Diagnosis/indication for surgery (n), patients | ||
| C. Mamma | 15 | 16 |
| DCIS | 2 | 0 |
| Prophylactic surgery | 1 | 2 |
| Duration of surgery (mean), min | 304.8 | 383.4 |
| Mastectomy weight (mean), g | 338.1 | 591.8 |
| Hospitalization (mean), days | 4.2 | 3.2 |
| Sufficient peroperative ICG-A, n (%), flaps | 9 (47.4) | 8 (32.0) |
| Insufficient peroperative ICG-A, n (%), flaps | 10 (52.6) | 17 (68.0) |
| Flaps with insufficient perfusion by ICG-A and Intraoperative change in surgery, n (%), flaps | 9 of 19 (47.4) | 12 of 25 (48.0) |
| Healing without complications, n (%), flaps | 3 (33.3) | 3 (25.0) |
| Postop. complications, n (%), flaps | 6 (66.6) | 9 (75.0) |
| CD grade 1 | 2 | 3 |
| CD grade 2 | 2 | 1 |
| CD grade 3A | – | 1 |
| CD grade 3B | 2 | 4 |
| Flaps with insufficient perfusion on ICG-A and no change in surgery (n), flaps | 1 | 5 |
| Healing without complications | – | 3 |
| Postop. complications | 1 | 2 |
| CD grade 1 | 1 | – |
| CD grade 2 | – | – |
| CD grade 3A | – | 1 |
| CD grade 3B | – | 1 |
| Complications in flaps with sufficient perfusion on ICG-A, n (%), flaps | 3 of 9 (33.3) | 5 of 8 (62.5) |
| Healing without complications | 6 (66.6) | 3 (37.5) |
| Postop. complications | 3 (33.3) | 5 (62.5) |
| CD grade 1 | – | 3 |
| CD grade 2 | 2 | 2 |
| CD grade 3A | – | – |
| CD grade 3B | 1 | – |
| Surgical complications, overall, n (%), flaps | – | – |
| Total | 10 of 19 (52.6) | 14 of 25 (56.0) |
| CD surgical classification | ||
| Grade 1 | 3 (30.0) | 5 (35.7) |
| Grade 2 | 4 (40.0) | 3 (21.4) |
| Grade 3A | 0 | 2 (14.3) |
| Grade 3B | 3 (30.0) | 4 (28.6) |
| Fat necrosis flaps, n (%), flaps | – | 5 of 25 (20.0) |
| Grade 1 | 2 | |
| Grade 2 | 1 | |
| Grade 3 | 1 | |
| Grade 4 | 1 | |
| Incidence of fat necrosis in flaps with insufficient perfusion on ICG-A, n (%), flaps | – | 3 of 5 (60.0) |
| Incidence of fat necrosis of total number of flaps with insufficient perfusion on ICG-A, n (%), flaps | – | 5 of 17 (29.4) |
| Preoperative CTA vs. peroperative ICG-A assessment flaps, n (%), flaps | ||
| ICG-A equal to CTA | – | 16 of 25 (64.0) |
| ICG-A not equal to CTA | – | 9 of 25 (36.0) |
| Clinical consequence taken upon peroperative ICG-A | ||
| Change of perforator selection due to ICG-A | – | 5 (20.0) |
| Additional perforator selected due to ICG-A | – | 4 (16.0) |
| Postoperative outcome in flaps with change of/additional perforator selection due to ICG-A, n (%), flaps | ||
| Uneventful healing | – | 4 of 9 (44.4) |
| Eventful healing | – | 5 of 9 (55.6) |
| CD grade 1 | – | 3 |
| CD grade 2 | – | 2 |
| Incidence of fat necrosis in flaps with change of/additional perforator based on ICG-A (n), flaps | – | 1 |
CD classification: grade 1: any deviation from the normal postoperative course without the need for pharmacological or surgical treatment. Grade 2: complications requiring pharmacological treatment with drugs. Grade 3: complications requiring surgical, endoscopic or radiological intervention: Grade 3A: surgery under local anesthesia; grade 3B: surgery under general anesthesia. pLD, pedicled latissimus dorsi; DIEP, deep inferior epigastric perforator; No., number; BMI, body mass index; C. Mamma, cancer mammae; DCIS, ductal carcinoma in situ; ICG-A, indocyanine green angiography; CD, Clavien-Dindo; Postop., postoperative; CTA, computed tomography angiography.
pLD flap breast reconstruction
Eighteen patients underwent a pLD-flap breast reconstruction, 1 was bilateral and 17 were unilateral procedures, comprising a total of 19 flaps (Figure 5, Table 2).

Peroperative ICG-A results and postoperative complications
The peroperative ICG-A perfusion assessment and postoperative complications were analyzed in Tables 2,3. Figure 6 illustrates the rate of postoperative complications according to sufficient/insufficient ICG-A +/− intraoperative revision.
Table 3
| ICG-A results and complications according to type of flap | pLD-flaps | DIEP-flaps | Overall (pLD + DIEP) |
|---|---|---|---|
| Sufficient vs. insufficient ICG-A and overall complications | 0.17; (0.02–2.03) | >0.99; (0.12–7.99) | 0.23; (0.11–0.18) |
| Insufficient ICG with intraoperative change vs. insufficient ICG-A and no change | 0.51; (0.0–11.1) | 0.6; (0.04–0.13) | 0.36; (0.25–23.7) |
| Sufficient vs. insufficient ICG-A and complications on recipient site | >0.99; (0.007–11.9) | 0.63; (0.006–4.2) | 0.20; (0.07–1.73) |
Data are presented as: P value; (95% CI). ICG-A, indocyanine green angiography; pLD, pedicled latissimus dorsi; DIEP, deep inferior epigastric perforator; CI, confidence interval.
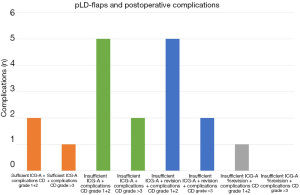
Table 4 shows the categorized complications based on the CD-classification.
Table 4
| Grade | Complications |
|---|---|
| CD grade 1 | Dry necrosis, seromas, wound dehiscence |
| CD grade 2 | Infections requiring antibiotic treatment, infected seromas, FN |
| CD grade 3A | Seromas and FN requiring surgical treatment |
| CD grade 3B | Hematomas, chronic seromas, skin necrosis, FN, partial flap loss, loss of implant |
Grade 1: any deviation from the normal postoperative course without the need for pharmacological or surgical treatment. Grade 2: complications requiring pharmacological treatment with drugs. Grade 3: complications requiring surgical, endoscopic or radiological intervention: Grade 3A surgery under local anesthesia; grade 3B surgery under general anesthesia. CD, Clavien-Dindo; FN, fat necrosis.
Statistical analysis was performed to investigate any association between peroperative ICG-A and postoperative complications Table 3. There was no significant association between peroperative ICG-A result and postoperative complications [P=0.17, 95% confidence interval (CI): 0.02–2.03]. Insufficient ICG-A results and the action thereof did not show any significant association to complications (P=0.51, 95% CI: 0.0–11.1). Also, when analyzing only the recipient site, there was no significant correlation between ICG-A and complications (P>0.99, 95% CI: 0.007–11.9).
Postoperative follow-up, BREAST-Q and POSAS
BREAST-Q and POSAS pre- and postoperative scores were analyzed Table 5.
Table 5
| Variables | pLD-flaps | DIEP-flaps |
|---|---|---|
| Lymphedema (n), patients | ||
| Baseline preop. | 7 | 5 |
| 4 weeks postop. | 7 | 6 |
| 4–6 months postop. | 7 | 6 |
| 12 months postop. | 7 | 6 |
| Adjuvant treatment on time (n), patients | ||
| On time | 14 (93.3%) | 16 (100%) |
| Delayed | 1 (due to GI-infection) | |
| BREAST-Q completed (%) | ||
| Preop. | 100† | 94 |
| 4 weeks postop. | 94† | 100‡ |
| 4–6 months postop. | 94† | 94‡ |
| 12 months postop. | 94† | 100‡ |
| POSAS completed (%; P value)§ | ||
| 4 weeks postop. | 88.9; 0.047 | 94 (due to COVID-19); not significant |
| 4–6 months | 100; 0.037 | 100; 0.01 |
| 12 months | 94; 0.008 | 100; 0.02 |
†, no significant differences comparing scores from each follow-up; ‡, significant improved QoL: 4 weeks to 4–6 months: P=0.04; 4–6 months to 12 months: P=0.01; §, P values indicate a significant worse scar scoring by the patient compared to the observer. pLD, pedicled latissimus dorsi; DIEP, deep inferior epigastric perforator; preop., preoperative; postop, postoperative; GI, gastrointestinal; POSAS, Patient and Observer Scar Assessment Score; QoL, quality of life; COVID-19, coronavirus disease 2019.
Pre- and postoperative scores revealed no significant difference. Also, no significant difference was found comparing scores from each follow-up.
Compared to the observer, patients scored their scars significantly worse at each postoperative assessment (Table 5).
Assessment of postoperative lymphedema
Six patients had undergone previous axillary lymph node dissection and 1 sentinel node biopsy. Five of the patients with lymphedema at baseline had been treated with adjuvant chemotherapy and postoperative radiation therapy. One received neo-adjuvant chemotherapy and 1 neo-adjuvant chemotherapy and postoperative radiation therapy.
At 12 months follow-up lymphedema was still present in the same 7 patients, 3 had experienced an improvement (decreased lymphedema of the upper extremity) both subjectively and when measured by the physiotherapist.
One patient reported that the lymphedema had worsened and 3 reported an unchanged state.
Treatment of lymphedema consisted of compression therapy and manual drainage. No patients developed lymphedema subsequent to the breast reconstruction.
Administration of adjuvant therapy
Fifteen patients received adjuvant treatment, administered on time in 14 patients (93.3%). One patient developed a postoperative gastrointestinal infection delaying antibody treatment (trastuzumab) for 1 week.
DIEP-flap breast reconstruction
Eighteen patients underwent DIEP-flap breast reconstruction resulting in 25 flaps. Eleven patients received unilateral procedures and 7 were bilateral (Figure 5, Table 2).
Peroperative ICG-A results and postoperative complications
The peroperative ICG-A perfusion assessment and postoperative complications were analyzed in Tables 2,3. Figure 7 illustrates the rate of postoperative complications according to sufficient/insufficient ICG-A +/− intraoperative revision.
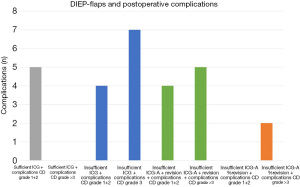
Table 4 shows the categorized complications based on the CD-classification.
Statistical analysis of peroperative ICG-A and the association to overall postoperative complications (P>0.99, 95% CI: 0.12–7.99), intraoperative change due to insufficient angiographies (P=0.6, 95% CI: 0.04–0.13) and complications at the recipient side (P=0.63, 95% CI: 0.006–4.2), showed no significant results Table 3.
Preoperative CTA vs. peroperative ICG-A assessment
Evaluation and selection of perforators were performed by preoperative CTA and peroperative ICG-A using the SPY-Elite®Table 2.
Postoperative follow-up, BREAST-Q and POSAS
All patients completed the 12 months follow-up. BREAST-Q and POSAS pre- and postoperative scores were analyzed in Table 5.
BREAST-Q significantly improved from 4 weeks to 4–6 months (95% CI: 0.17–18.5, P=0.04), and from 4–6 months to 12 months (95% CI: 0.91–5.76, P=0.01).
Compared to the observer, patients scored their scars significantly worse at the 4–6 months (W =104, P=0.01) and 12 months assessment (W =89, P=0.02).
Assessment of postoperative lymphedema
Preoperatively, 1 patient reported that she suspected incipient lymphedema of the upper extremity. She had previously been treated for bilateral breast cancer. No lymphedema was diagnosed objectively, but clinical presence of lymphedema became present at the 12 months evaluation.
Of the 6 patients who had lymphedema after 12 months, all had previously undergone axillary lymph node dissection, and received adjuvant treatment consisting of chemotherapy and radiation.
During the 12 months follow-up, 2 patients reported an improvement of lymphedema, 1 experienced a worsened stated and 3 had unchanged state. Treatment of lymphedema consisted of compression therapy and manual drainage.
No patients developed lymphedema subsequent to the breast reconstruction.
Administration of adjuvant therapy
Sixteen patients received adjuvant treatment, administered on time in all patients.
Discussion
Complications following breast reconstructive surgery—and especially major/higher grade complications—remain an important issue. Postoperative complication rates, regardless of the method or timing, have been reported to be as high as 50% (40,41). These complications can range from mild issues like delayed wound healing to severe such as partial or total flap loss (40,42,43). Despite the relatively frequent occurrence of complications, there is currently no effective method for real-time monitoring of tissue perfusion, highlighting the need for more accurate assessment methods (31,44,45).
In our study, we observed an overall major surgical complication rate (CD >3) of 15.8% in the pLD-group and 24% in the DIEP-group, which is consistent with findings from other studies (27,46-49). Also, complication rates in both groups are comparable to historical cohorts from our center (50,51).
Peroperative ICG-A measurements and postoperative complications
Peroperative ICG-A is associated with a decreased risk of complications in both implant-based and ABRs (25,26,52). Also, ICG-A have been investigated for peroperative flap design and perforator mapping (53-55) with conflicting results (19,54,56). A consensus study from 2022 concluded ICG-A to be an integrated and valuable tool in perfusion assessment (28), but details on application, assessment and interpretation remain unstandardized (16,57,58).
Despite intraoperative modifications in response to ICG-A results, more than two-thirds of patients in this study experienced postoperative complications. Statistical analysis comparing complication rates with insufficient perfusion +/− intraoperative change, revealed no significant difference, possibly due to sample size.
This current study had an observational design, and it is important to note that there was a significant learning curve in the process of incorporating ICG-A into surgical procedures and responding to its findings. Over time, surgeons have developed enhanced skills in the intraoperative utilization of ICG-A, improved interpretation, and more refined decision-making during surgery which is reflected in the results.
Reflecting on the results, an insufficient intraoperative ICG-A may indicate compromised perfusion, and consequently intraoperative action needs to be taken upon the result (59,60). This study demonstrated the feasibility, application and utilization of intraoperative ICG-A in a broader setting involving multiple surgeons, and it has now become the standard practice for breast reconstructive procedures in our clinic.
Selection of perforators: DIEP-flap breast reconstruction
Studies examining the vascular anatomy, quality, size, and location of abdominal wall perforators are numerous (61,62). However, there is currently no consensus on the ideal DIEP-flap perfusion classification system (63).
Also, studies have explored the use of ICG-A in optimizing flap design, perforator selection, and location (49,53-55). Park et al. used ICG-A to analyze DIEP-flap perfusion based on the vertical location of dominant perforators and found that adding an additional perforator improved perfusion (19). Min et al. investigated single perforator DIEP-flaps and contralateral perfusion using ICG-A (64).
In our study, peroperative ICG-A led to a change in the selected perforator in 20% of cases, and an additional perforator was added in 16% of all flaps. Approximately 56% of these cases experienced postoperative complications. However, due to the small sample size, definitive conclusions cannot be drawn from these findings.
FN: DIEP-flap breast reconstruction
FN is a common complication in ABR and can result from insufficient perfusion after vascular anastomosis, leading to both complete and partial necrosis, which can be devastating for the patient (65).
Reported rates of FN in previous studies have varied widely, ranging from 3.4% to 59.5% (16,23,66,67). Studies investigating DIEP-flap procedures with and without the use of intraoperative ICG-A have consistently shown an association between ICG-A and a reduced risk of FN (16,19,23,25,26,49,56,66-68). One study by Casey et al. reported the most significant reduction, with FN decreasing from 71.4% to 0% when ICG-A was used (68).
The findings of this study align with previous reports, indicating that FN rates are influenced by ICG-A use, highlighting its potential to reduce the risk of this complication.
CTA vs. ICG-A: DIEP-flap breast reconstruction
Preoperative CTA is considered the gold standard for planning DIEP-flap breast reconstruction (32). CTA offers advantages like preoperative perforator identification and insights into the superficial system and deep inferior epigastric pedicle anatomy. Nonetheless, it has drawbacks, including radiation exposure and contrast load (69).
In contrast, ICG-A provides real-time information on perforators and perfusion. In this study, the agreement between perforators identified by CTA and ICG-A was 64%, consistent with previous research reporting a 67.3% concordance between radiologist-identified perforators and surgeon-chosen perforators (32).
Pestana et al. assessed the correlation between ICG-A and CTA in vessel identification, as well as the correlation with perforator vessel size and number. They did not find a significant correlation between the two methods (70).
In summary, while CTA remains a valuable tool for DIEP-flap breast reconstruction planning, ICG-A offers real-time information on perforators and perfusion, and their concordance varies in different studies.
Postoperative follow up
The primary goal of reconstructive breast surgery is to enhance patients’ QoL. QoL is important because morbidity and mortality alone does not suffice as a measurement of breast reconstructive success. Patient self-evaluation becomes valuable in assessing surgical outcomes, considering that patients and surgeons may perceive pre- and postoperative states differently.
In this study, BREAST-Q and POSAS assessments were conducted at postoperative visits. In the pLD-group, no significant differences in BREAST-Q scores were observed across follow-up assessments. In the DIEP-group, QoL significantly improved over the 12-month follow-up period, with notable enhancements between 4 weeks to 4–6 months and 4–6 months to 12 months. Nevertheless, when comparing pre- and postoperative scores within the DIEP-group, no significant change was noted.
With regard to scar assessments, patients consistently rated their scars significantly worse than observer assessments, highlighting the divergence between patient and surgeon perspectives on scar appearance.
Breast cancer-related lymphedema is a chronic condition associated with various adverse effects, was also assessed. Risk factors included axillary lymph node dissection and radiotherapy. A specialized physiotherapist conducted postoperative lymphedema assessments, revealing that all 13 patients with lymphedema before breast reconstruction still had lymphedema at the 12-month follow-up. Among them, 5 patients reported improvement, while only 2 reported worsening. However, the limited number of cases precludes drawing definitive conclusions or identifying trends, despite prior studies suggesting a potential link between breast reconstruction and reduced lymphedema risk. Importantly, though lymphedema can occur from months to years after breast cancer treatment (71), no patients developed lymphedema after the reconstructive procedure within the 1-year follow-up of this study.
Limitations
The study is conducted as a prospective observational study.
To minimize interobserver bias, the intraoperative application and interpretation of the peroperative ICG-A was performed by the same operator.
Patient characteristics, complications and clinical intraoperative perfusion assessment by the operating surgeon(s) were documented prospectively avoiding recall- and reporting bias. The CD classification system for surgical complications was applied to standardize the assessment and reporting of complications.
The study is limited due to the relatively small sample size. To strengthen analysis and level of evidence, further studies could be performed as, e.g., randomized superiority clinical trial including a larger sample size.
Conclusions
Peroperative use of ICG-A was utilized for ABRs involving pLD- and DIEP-flaps. Insufficient tissue perfusion detected through peroperative ICG-A measurements influenced surgical decision-making in 50%. Notably, in 36% of DIEP-flaps, the selection of perforator(s) was modified due to inconsistencies between preoperative CTA and peroperative ICG-A results.
Despite the observational design of this study, we successfully demonstrated the feasibility of implementing multiple ICG-A assessments during ABR, thereby paving the way for future investigations with higher levels of evidence.
Larger prospective studies with robust study designs, such as randomized clinical trials, are needed to obtain higher-quality data and draw more definitive conclusions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-23-53/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-23-53/dss
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-23-53/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-23-53/coif). T.E.D. serves as an unpaid editorial board member of Annals of Breast Surgery from July 2023 to June 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Capital Denmark Region Committees on Health Research Ethics (H-19074897, 70432) and conducted in accordance with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Breast cancer. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer
- Uroskie TW, Colen LB. History of breast reconstruction. Semin Plast Surg 2004;18:65-9. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Christiansen P, Mele M, Bodilsen A, et al. Breast-Conserving Surgery or Mastectomy?: Impact on Survival. Ann Surg Open 2022;3:e205. [Crossref] [PubMed]
- Bertozzi N, Pesce M, Santi PL, et al. Oncoplastic breast surgery: comprehensive review. Eur Rev Med Pharmacol Sci 2017;21:2572-85. [PubMed]
- von Glinski M, Holler N, Kümmel S, et al. Autologous vs. implant-based breast reconstruction after skin- and nipple-sparing mastectomy-A deeper insight considering surgical and patient-reported outcomes. Front Surg 2022;9:903734. [Crossref] [PubMed]
- Tansini I. Sopra il mio nuovo processo di amputazione della mamella Riforma Med 1906;12:757. [in Italian].
- Schneider WJ, Hill HL Jr, Brown RG. Latissimus dorsi myocutaneous flap for breast reconstruction. Br J Plast Surg 1977;30:277-81. [Crossref] [PubMed]
- Bostwick J 3rd, Vasconez LO, Jurkiewicz MJ. Breast reconstruction after a radical mastectomy. Plast Reconstr Surg 1978;61:682-93. [Crossref] [PubMed]
- Papp C, McCraw JB. Autogenous latissimus breast reconstruction. Clin Plast Surg 1998;25:261-6. [Crossref] [PubMed]
- Spear S, Clemens M. Latissimus dorsi flap breast reconstruction. In: Neligan PC, Grotting JC, editors. Plastic Surgery. 3rd ed. Philadelphia, PA: Saunders (Elsevier); 2012:370-92.
- Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg 1979;13:423-7. [Crossref] [PubMed]
- Chang DW. Breast Reconstruction with Microvascular MS-TRAM and DIEP Flaps. Arch Plast Surg 2012;39:3-10. [Crossref] [PubMed]
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Momeni A, Sheckter C. Intraoperative Laser-Assisted Indocyanine Green Imaging Can Reduce the Rate of Fat Necrosis in Microsurgical Breast Reconstruction. Plast Reconstr Surg 2020;145:507e-13e. [Crossref] [PubMed]
- Healy C, Allen RJ Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg 2014;30:121-5. [PubMed]
- Lee KT, Eom Y, Jeon BJ, et al. Vertical Spacing of Perforators in Deep Inferior Epigastric Perforator Flap Breast Reconstruction Can Affect the Outcomes. Plast Reconstr Surg 2018;142:319-29. [Crossref] [PubMed]
- Park JW, Lee MK, Woo KJ. Influence of vertical location and spacing of perforators on perfusion in deep inferior epigastric artery perforator flap breast reconstruction: quantitative analysis using indocyanine green angiography. Gland Surg 2022;11:1851-63. [Crossref] [PubMed]
- Cai A, Suckau J, Arkudas A, et al. Autologous Breast Reconstruction with Transverse Rectus Abdominis Musculocutaneous (TRAM) or Deep Inferior Epigastric Perforator (DIEP) Flaps: An Analysis of the 100 Most Cited Articles. Med Sci Monit 2019;25:3520-36. [Crossref] [PubMed]
- Pollhammer MS, Duscher D, Schmidt M, et al. Recent advances in microvascular autologous breast reconstruction after ablative tumor surgery. World J Clin Oncol 2016;7:114-21. [Crossref] [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [Crossref] [PubMed]
- Parmeshwar N, Sultan SM, Kim EA, et al. A Systematic Review of the Utility of Indocyanine Angiography in Autologous Breast Reconstruction. Ann Plast Surg 2021;86:601-6. [Crossref] [PubMed]
- Holm C, Mayr M, Höfter E, et al. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg 2002;55:635-44. [Crossref] [PubMed]
- Lauritzen E, Damsgaard TE. Use of Indocyanine Green Angiography decreases the risk of complications in autologous- and implant-based breast reconstruction: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2021;74:1703-17. [Crossref] [PubMed]
- Varela R, Casado-Sanchez C, Zarbakhsh S, et al. Outcomes of DIEP Flap and Fluorescent Angiography: A Randomized Controlled Clinical Trial. Plast Reconstr Surg 2020;145:1-10. [Crossref] [PubMed]
- Alstrup T, Christensen BO, Damsgaard TE. ICG angiography in immediate and delayed autologous breast reconstructions: peroperative evaluation and postoperative outcomes. J Plast Surg Hand Surg 2018;52:307-11. [Crossref] [PubMed]
- Schols RM, Dip F, Lo Menzo E, et al. Delphi survey of intercontinental experts to identify areas of consensus on the use of indocyanine green angiography for tissue perfusion assessment during plastic and reconstructive surgery. Surgery 2022;172:S46-53. [Crossref] [PubMed]
- Schaafsma BE, Mieog JS, Hutteman M, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol 2011;104:323-32. [Crossref] [PubMed]
- Zenn MR. Fluorescent angiography. Clin Plast Surg 2011;38:293-300. [Crossref] [PubMed]
- Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg 2012;129:1043-8. [Crossref] [PubMed]
- Boer VB, van Wingerden JJ, Wever CF, et al. Concordance between preoperative computed tomography angiographic mapping and intraoperative perforator selection for deep inferior epigastric artery perforator flap breast reconstructions. Gland Surg 2017;6:620-9. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Willert CB, Gjørup CA, Hölmich LR. Danish translation and linguistic validation of the BREAST-Q. Dan Med J 2020;67:A08190445. [PubMed]
- van de Kar AL, Corion LU, Smeulders MJ, et al. Reliable and feasible evaluation of linear scars by the Patient and Observer Scar Assessment Scale. Plast Reconstr Surg 2005;116:514-22. [Crossref] [PubMed]
- Kilgore LJ, Korentager SS, Hangge AN, et al. Reducing Breast Cancer-Related Lymphedema (BCRL) Through Prospective Surveillance Monitoring Using Bioimpedance Spectroscopy (BIS) and Patient Directed Self-Interventions. Ann Surg Oncol 2018;25:2948-52. [Crossref] [PubMed]
- The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020;53:3-19. [PubMed]
- Lie KH, Barker AS, Ashton MW. A classification system for partial and complete DIEP flap necrosis based on a review of 17,096 DIEP flaps in 693 articles including analysis of 152 total flap failures. Plast Reconstr Surg 2013;132:1401-8. [Crossref] [PubMed]
- Mundy LR, Homa K, Klassen AF, et al. Breast Cancer and Reconstruction: Normative Data for Interpreting the BREAST-Q. Plast Reconstr Surg 2017;139:1046e-55e. [Crossref] [PubMed]
- Sullivan SR, Fletcher DRD, Isom CD, et al. True incidence of all complications following immediate and delayed breast reconstruction. Plast Reconstr Surg 2008;122:19-28. [Crossref] [PubMed]
- Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg 2002;109:2265-74. [Crossref] [PubMed]
- Patel KM, Hill LM, Gatti ME, et al. Management of massive mastectomy skin flap necrosis following autologous breast reconstruction. Ann Plast Surg 2012;69:139-44. [Crossref] [PubMed]
- Andrades P, Fix RJ, Danilla S, et al. Ischemic complications in pedicle, free, and muscle sparing transverse rectus abdominis myocutaneous flaps for breast reconstruction. Ann Plast Surg 2008;60:562-7. [Crossref] [PubMed]
- Munabi NC, Olorunnipa OB, Goltsman D, et al. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg 2014;67:449-55. [Crossref] [PubMed]
- Newman MI, Samson MC, Tamburrino JF, et al. Intraoperative laser-assisted indocyanine green angiography for the evaluation of mastectomy flaps in immediate breast reconstruction. J Reconstr Microsurg 2010;26:487-92. [Crossref] [PubMed]
- Mirhaidari SJ, Beddell GM, Orlando MV, et al. A Prospective Study of Immediate Breast Reconstruction with Laser-Assisted Indocyanine Green Angiography. Plast Reconstr Surg Glob Open 2018;6:e1774. [Crossref] [PubMed]
- Leuzzi S, Stivala A, Shaff JB, et al. Latissimus dorsi breast reconstruction with or without implants: A comparison between outcome and patient satisfaction. J Plast Reconstr Aesthet Surg 2019;72:381-93. [Crossref] [PubMed]
- Agaoglu G, Erol OO. Delayed breast reconstruction with latissimus dorsi flap. Aesthetic Plast Surg 2009;33:413-20. [Crossref] [PubMed]
- Hembd A, Teotia SS, Zhu H, et al. Optimizing Perforator Selection: A Multivariable Analysis of Predictors for Fat Necrosis and Abdominal Morbidity in DIEP Flap Breast Reconstruction. Plast Reconstr Surg 2018;142:583-92. [Crossref] [PubMed]
- Bonde CT, Højvig JB, Jensen LT, et al. Long-term results of a standardized enhanced recovery protocol in unilateral, secondary autologous breast reconstructions using an abdominal free flap. J Plast Reconstr Aesthet Surg 2022;75:1117-22. [Crossref] [PubMed]
- Højvig JB, Bonde CT. Breast reconstruction using a latissimus dorsi flap after mastectomy. Dan Med J 2015;62:A5155. [PubMed]
- Pruimboom T, Schols RM, Van Kuijk SM, et al. Indocyanine green angiography for preventing postoperative mastectomy skin flap necrosis in immediate breast reconstruction. Cochrane Database Syst Rev 2020;4:CD013280. [Crossref] [PubMed]
- Ludolph I, Arkudas A, Schmitz M, et al. Cracking the perfusion code?: Laser-assisted Indocyanine Green angiography and combined laser Doppler spectrophotometry for intraoperative evaluation of tissue perfusion in autologous breast reconstruction with DIEP or ms-TRAM flaps. J Plast Reconstr Aesthet Surg 2016;69:1382-8. [Crossref] [PubMed]
- Girard N, Delomenie M, Malhaire C, et al. Innovative DIEP flap perfusion evaluation tool: Qualitative and quantitative analysis of indocyanine green-based fluorescence angiography with the SPY-Q proprietary software. PLoS One 2019;14:e0217698. [Crossref] [PubMed]
- Chirappapha P, Chansoon T, Bureewong S, et al. Is It Reasonable to Use Indocyanine Green Fluorescence Imaging to Determine the Border of Pedicled TRAM Flap Zone IV? Plast Reconstr Surg Glob Open 2020;8:e3093. [Crossref] [PubMed]
- Malagón-López P, Carrasco-López C, García-Senosiain O, et al. When to assess the DIEP flap perfusion by intraoperative indocyanine green angiography in breast reconstruction? Breast 2019;47:102-8. [Crossref] [PubMed]
- Hembd AS, Yan J, Zhu H, et al. Intraoperative Assessment of DIEP Flap Breast Reconstruction Using Indocyanine Green Angiography: Reduction of Fat Necrosis, Resection Volumes, and Postoperative Surveillance. Plast Reconstr Surg 2020;146:1e-10e. [Crossref] [PubMed]
- Lauritzen E, Bredgaard R, Bonde C, et al. An observational study comparing the SPY-Elite® vs. the SPY-PHI QP system in breast reconstructive surgery. Ann Breast Surg 2023;7:12. [Crossref]
- Yoshimatsu H, Karakawa R, Scaglioni MF, et al. Application of intraoperative indocyanine green angiography for detecting flap congestion in the use of free deep inferior epigastric perforator flaps for breast reconstruction. Microsurgery 2021;41:522-6. [Crossref] [PubMed]
- Yoshimatsu H, Karakawa R, Scaglioni MF, et al. Use of intraoperative indocyanine green angiography for detection and prediction of congestion in pedicled island flaps. Microsurgery 2023;43:452-9. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Le Roux CM, et al. The perforator angiosome: a new concept in the design of deep inferior epigastric artery perforator flaps for breast reconstruction. Microsurgery 2010;30:1-7. [Crossref] [PubMed]
- Saint-Cyr M, Wong C, Schaverien M, et al. The perforasome theory: vascular anatomy and clinical implications. Plast Reconstr Surg 2009;124:1529-44. [Crossref] [PubMed]
- Ludolph I, Bettray D, Beier JP, et al. Leaving the perfusion zones? Individualized flap design in 100 free DIEP and ms-TRAM flaps for autologous breast reconstruction using indocyanine green angiography. J Plast Reconstr Aesthet Surg 2022;75:52-60. [Crossref] [PubMed]
- Min K, Oh SM, Kim EK, et al. Analysis of Perfusion in the DIEP Flap: Role of the Location of the Perforator, Umbilicus, and Midline Crossing-Over Vessel. Plast Reconstr Surg 2023;151:1146-55. [Crossref] [PubMed]
- Wang XL, Liu LB, Song FM, et al. Meta-analysis of the safety and factors contributing to complications of MS-TRAM, DIEP, and SIEA flaps for breast reconstruction. Aesthetic Plast Surg 2014;38:681-91. [Crossref] [PubMed]
- Wagner IJ, Tong WM, Halvorson EG. A classification system for fat necrosis in autologous breast reconstruction. Ann Plast Surg 2013;70:553-6. [Crossref] [PubMed]
- Malagón-López P, Vilà J, Carrasco-López C, et al. Intraoperative Indocyanine Green Angiography for Fat Necrosis Reduction in the Deep Inferior Epigastric Perforator (DIEP) Flap. Aesthet Surg J 2019;39:NP45-54. [Crossref] [PubMed]
- Casey WJ 3rd, Connolly KA, Nanda A, et al. Indocyanine green laser angiography improves deep inferior epigastric perforator flap outcomes following abdominal suction lipectomy. Plast Reconstr Surg 2015;135:491e-7e. [Crossref] [PubMed]
- Chang EI, Chu CK, Chang EI. Advancements in imaging technology for microvascular free tissue transfer. J Surg Oncol 2018;118:729-35. [Crossref] [PubMed]
- Pestana IA, Zenn MR. Correlation between abdominal perforator vessels identified with preoperative CT angiography and intraoperative fluorescent angiography in the microsurgical breast reconstruction patient. Ann Plast Surg 2014;72:S144-9. [Crossref] [PubMed]
- Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol 2009;27:390-7. [Crossref] [PubMed]
Cite this article as: Lauritzen E, Bredgaard R, Bonde C, Jensen LT, Tvedskov T, Damsgaard TE. Indocyanine green angiography for autologous breast reconstruction: a prospective observational study. Ann Breast Surg 2024;8:17.

