Barcelona lymphedema algorithm for surgical treatment (BLAST)
Introduction
Female breast cancer, with an estimated 2.3 million new cases diagnosed in 2020, remains the most commonly diagnosed cancer worldwide (1). After breast cancer treatment, upper limb lymphedema is a chronic and progressive sequela that impairs patients’ quality of life (2,3). The overall estimated incidence of breast cancer-related lymphedema (BCRL) is around 21% but, due to the lack of diagnostic criteria, ranges from as much as 5% to 30% (4). Because long-term survival rates in women with proper breast cancer treatment are as high as 80% at 15 years (5), adequate management of BCRL represents a major challenge for both patients and healthcare professionals.
BCRL typically results from the interruption of the lymphatic drainage produced by surgical dissection of the axillary lymph nodes. A well-established risk factor for this complication is regional lymph node radiation (6). Nevertheless, the incidence of BCRL may gradually be decreased by current trends in less invasive axillary therapy in patients with a positive sentinel node biopsy (7) and less aggressive radiotherapy protocols (8-10).
Traditional treatment for patients with BCRL has typically involved conservative methods including a combination of manual lymphatic drainage, compression garments, exercises, and skin care (11). However, the efficacy of these therapies alone is limited, as the existence of structural damage to the lymphatic system continues to impede adequate lymph drainage of the affected upper limb.
Over the last five decades, our understanding of the anatomy and pathophysiology of the lymphatic system has been enhanced by advances in imaging techniques and higher microscope magnification, leading to the development of various surgical techniques for the treatment of BCRL. However, the surgical management of BCRL is constantly evolving and there is no established consensus on the optimal treatment of these patients. Consequently, we would like to share an update of the therapeutic algorithm used in our centers, which has yielded highly encouraging results and could potentially be used to guide decision-making when planning the surgical treatment of BCRL.
Clinical considerations
According to the International Society of Lymphology (ISL), accurate clinical history and physical examination are essential for a proper diagnosis of BCRL and its staging (Table 1) (12). The main clinical manifestation of lymphedema is swelling of part or all of the limb, which may be accompanied by sensations of tightness, heaviness or fullness, and sometimes by pain in the affected area.
Table 1
| ISL stage | Features |
|---|---|
| 0 | Latent or sub-clinical condition where swelling is not evident despite impaired lymph transport. It may exist months or years before overt edema occurs |
| I | Early accumulation of fluid relatively high in protein content and subsides with limb elevation. Pitting may occur |
| II | Limb elevation alone rarely reduces tissue swelling and pitting is manifest. Late in Stage II, the limb may or may not pit as tissue fibrosis supervenes |
| III | Lymphostatic elephantiasis where pitting is absent and trophic skin changes such as acanthosis, fat deposits, and warty overgrowths develop |
ISL, International Society of Lymphology.
On physical examination, it is important to distinguish between pitting and non-pitting edema. Swelling in the early stages of lymphedema is characterized by the presence of pitting, resulting from the accumulation of protein-rich fluid in the interstitial space. Subsequently, as the inadequate lymph drainage persists, the continued state of lymph overload leads to a failure of the lymphatic pump (13). Consequently, the permanent lymph stasis generates a chronic inflammatory response, inducing progressive degeneration of functional lymphatic channels (from the site of the interruption to the distal area), and a proliferation of adipose and connective subcutaneous tissue (14-16). Thus, as lymphedema progresses, upper extremity swelling will transition from pitting to non-pitting edema due to progressive hypertrophy of the subcutaneous tissue. Therefore, the more advanced stages of lymphedema are characterized by the absence of pitting, which is often accompanied by the presence of trophic skin changes, and the increased arm volume may even restrict range of motion.
Clinical assessment should also include measurement of limb circumference and volume. Girth measurements may be obtained by multiple circumference measurements of both limbs using a spring-loaded tape measure (5-cm intervals starting at the elbow, then progressing down to the dorsum of the hand, and then up to the shoulder). Volume measurements can be obtained using circumference measurements through the truncated cone model (17). However, other methods can also be used, including plethysmography and perometer. Based on the difference in limb volume, severity can be assessed as minimal (<20% increase), moderate (20–40% increase), or severe (>40% increase) (12). Understanding the clinical manifestations and pathophysiological processes of lymphedema is essential to comprehend the principles underlying the surgical treatment of this disease.
Diagnostic imaging techniques
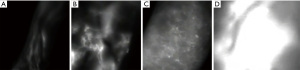
After clinical diagnosis and ISL staging, imaging techniques play an essential role in assessing the structural and functional features of the lymphatic system. One of the main diagnostic imaging techniques is indocyanine green lymphography (ICG-L), which can be used to assess the functionality of the superficial lymphatic system. The findings can be classified into linear or dermal backflow patterns. Linear patterns correspond to normal active subdermal lymphatic channels (up to a depth of 1.5 to 2 cm), while nonlinear or dermal backflow patterns represent the accumulation of lymphatic fluid in the interstitial space. Dermal backflow patterns can be further divided into splash, stardust, and diffuse patterns, which correspond to the degree of severity of lymphedema (Figure 1) (18,19).
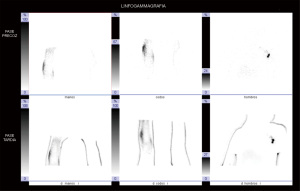
The other main diagnostic imaging technique for the assessment of the lymphatic system is lymphoscintigraphy (LS). This imaging modality allows qualitative assessment of the functionality of the deep lymphatic system. Among the main parameters evaluated are tracer uptake and migration speed, visualization of major lymphatic collectors and axillary lymph node basins, as well as the time taken by the tracer to reach them, and calculation of the transport index (Figure 2) (20).
Generally, ICG-L and LS provide sufficient information to determine the most appropriate surgical strategy. However, a third imaging technique, magnetic resonance lymphography (MRL), has been introduced in the last decade, which we request in our center to complement certain limitations of the two previous imaging techniques or resolve discrepancies between their findings. MRL provides three-dimensional high-resolution anatomic images of the superficial and deep lymphatic systems, including lymph node basins, as well as useful information on the function of the lymphatic system. This imaging modality also provides detailed characterization of lymphedema-associated soft tissue changes and detailed limb circumference measurements that can be used to calculate limb volume (21,22). More recently, ultra-high frequency ultrasound (UHFUS) has also been introduced, which provides more acurate imaging of the structure of the subdermal lymphatic vessels. This technique can even detect the lymphatic vessels, where dermal backflow patterns were revealed by ICG-L. However, acquisition of accurate images by UHFUS is highly operator-dependent (23,24).
Surgical techniques
Surgical techniques for BCRL treatment can be divided into physiologic procedures, which attempt to re-establish lymphatic drainage and increase lymphatic fluid clearance, and ablative procedures, which aim to remove excess subcutaneous tissue in order to reduce limb volume. Currently, the three main pillars for the surgical treatment of BCRL are lymphatic-venous anastomosis (LVA), functional vascularized lymphatic tissue transfer (FVLTT), and liposuction (25). The three techniques have different therapeutic aims and their indications depend on the pathophysiological changes of the affected upper limb.
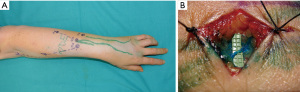
LVA is a supermicrosurgical physiologic procedure that connects lymphatic channels to nearby subdermal veins to redirect lymph drainage to the venous system in the limb (26). We recommend the use of this technique when functional lymphatic channels remain throughout the limb. Patient suitability for LVA can usually be determined by ICG-L. ICG-L or MRL can be used for preoperative planning. However, the combination of these two techniques allows for better location and selection of the most suitable functional lymphatic channels for LVA (Figure 3) (27). Moreover, if UHFUS is available, it may be employed for a more accurate preoperative identification of the subdermal lymphatic channels and nearby veins to be anastomosed (23,24).
FVLTT is a microsurgical physiologic procedure involving the transfer of a vascularized flap with a functional lymphatic network. This functional lymphatic tissue flap is transferred from another region of the body to an area where the native lymph node basins and/or lymphatic channels are no longer functional. The exact mechanism of FVLTT is still under debate. One hypothesis is that the transferred functional lymphatic tissue may induce lymphangiogenesis and act as a wick to bridge gaps between the proximal and distal lymphatic vessels in the recipient site (28,29). The other hypothesis proposes that the vascularized lymphatic tissue acts as “lymph pumps”. These pumps absorb lymph fluid from the surrounding interstitial tissue and then expel it into the venous circulation by means of lymphovenous communication within the nodes in the transferred flap (29,30).
Various donor sites can be used for the FVLTT technique, with the most common being the iliac-inguinal region, with the superficial circumflex iliac vessels being used as the vascular pedicle. When planning FVLTT through the use of a groin flap, computed tomography angiography is needed to provide information on the location of the vascular pedicle of the flap and inguinal lymph nodes. In our practice, the recipient area for the FVLTT is the axillary region and proximal part of the limb (Figure 4). Both LS and ICG-L are usually needed to assess the functionality of the lymphatic system of the proximal part of the arm, and to determine whether the patient is suitable for FVLTT. In some cases, FVLTT can even be combined with autologous breast reconstruction. For this purpose, the ideal donor site is the lower abdominal region. For lymphatic tissue transfer, tissue is taken from the iliac-inguinal region, with the superficial circumflex iliac vessels providing a vascular pedicle. For breast reconstruction, tissue is taken from the lower abdomen, with the deep inferior epigastric vessels providing a pedicle. The two flaps are then harvested as one and positioned in the thorax (31). This combined reconstructive procedure is known as total breast anatomy restoration (TBAR) (32).
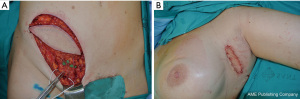
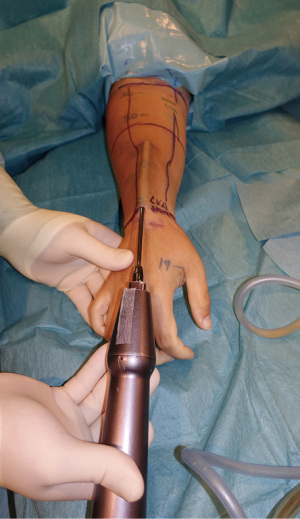
Despite the revolutionary concept of restoring the functionality of the lymphatic system together with effective maintenance decongestive therapy, neither of these techniques completely reduces limb circumference in more advanced stages of lymphedema, because the excess volume is mostly related to fat hypertrophy and fibrosis. In this context, liposuction is the preferred surgical procedure to remove excess subcutaneous tissue. This reductive technique helps to achieve similar circumference measurements to those of the contralateral limb, improve patient comfort, and reduce the incidence of erysipelas episodes (33). For this procedure, we recommend the use of power-assisted liposuction with vibrating cannulas (Figure 5). The liposuction technique is executed circumferentially from the wrist to the shoulder. Postoperative compression therapy is crucial to obtain favorable results (34).
The Barcelona lymphedema algorithm for surgical treatment (BLAST)
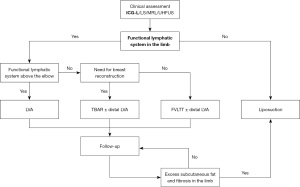
Because both the speed and severity of the pathophysiological changes of lymphedema are unpredictable and vary widely among patients, the surgical management of BCRL is complex. Therefore, the approach should be individualized based on the degree of involvement of the lymphatic system and hypertrophy of the subcutaneous tissue (32,35). The surgical strategy may often involve a combination of techniques, either in the same intervention or in two stages. Below, we summarize the main clinical scenarios that can arise, depending on the findings of diagnostic imaging techniques and clinical assessment, and our surgical approach to the treatment of BCRL (Figure 6).
- Functional lymphatic system above the elbow on ICG-L/MRL: these findings generally correspond to ISL stages I–II. We recommend performing the LVA technique to improve lymph drainage by redirecting the flow of the functional lymphatic channels to the venous circulation in the limb itself. Subsequent periodic follow-up is also needed to assess the response to treatment and outcomes.
- Functional lymphatic system up to or below the elbow on ICG-L/MRL: these findings usually correspond to ISL stage II. We recommend a combined surgical approach using the FVLTT technique to improve lymph drainage of the proximal part of the limb, and the LVA technique at the level of the functional lymphatic channels to improve lymph drainage of the distal part of the limb. This combined approach can be performed either in the same intervention or in two stages. It should also be considered if the patient needs a breast reconstruction, to assess the possibilities of performing the TBAR technique. Subsequent periodic follow-up is also needed to assess the response to treatment and outcomes.
- Non-functional lymphatic system on ICG-L/LS/MRL: these findings generally correspond to ISL stage III. In patients with a moderate (20–40% increase) or severe (>40% increase) difference in the volume of the limb, and a history of erysipelas, pain, or reduced arm mobility, we recommend liposuction.
- Patient with previous physiologic procedures (LVA, FVLTT ± LVA or TBAR ± LVA) and excess subcutaneous fat and fibrosis. In some patients who have undergone successful physiological surgical interventions, the volume of the limb may not decrease to that of the contralateral side. This is due to the development of excess subcutaneous fat and fibrosis. These patients may correspond to ISL late stage II. In patients with a moderate (20–40% increase) difference in the volume of the limb that affects the quality of life, we recommend performing liposuction as a complementary surgical procedure.
Risk reducing lymphedema surgery
The earlier reconstructive procedures are performed, the greater their effectiveness. Accordingly, the most recent trend is BCRL risk reducing surgery. This new approach involves intraoperative evaluation of the axillary lymphatic system in patients undergoing lymph node dissection and immediate surgical repair of the sectioned lymphatic channels.
This innovative approach was first reported by Boccardo et al. (36) and was named the lymphatic microsurgical preventive healing approach (LYMPHA). The surgical technique consists of injecting a blue dye to visualize the afferent lymphatic channels during axillary lymph node dissection and the sectioned lymphatic channels are then introduced inside the nearby veins cut end using a U-shaped stitch.
The reported incidence of BCRL with the LYMPHA was highly encouraging (4.34% in the LYMPHA group vs. 30.43% in the control group not undergoing surgical prevention) (37). However, some studies show that the lymphatic-venous implantation technique is associated with a very high rate of blood clot formation and subsequent blood vessel obstruction (38,39).
More recently, a similar surgical approach was developed by our team for the prevention of upper limb lymphedema secondary to breast cancer treatment, the targeted-lymphatic axillary repair (T-LAR) approach. This technique involves axillary reverse mapping using ICG-L and blue patent V dye to identify afferent lymphatic channels during axillary lymph node dissection. When interruption of lymphatic channel is confirmed, an immediate bypass is performed between the transected lymphatic channel and small tributaries of axillary veins through end-to-end lymphatic-venous anastomosis. Because of the high precision of axillary reverse mapping in identifying sectioned afferent lymphatic channels and the feasibility of lymphatic venous bypass through “true” anastomoses that maintain the continuity of the intima of the vessels, the T-LAR approach is a promising procedure to significantly reduce the incidence of BCRL (40).
Conclusions
The key to successful management of BCRL is optimal patient selection and individualized surgical treatment based on the structural and functional involvement of the lymphatic system. Screening for BCRL by ICG-L in patients with previous axillary lymph node dissection should be standard practice for early diagnosis and prompt surgical treatment. Nevertheless, risk-reducing lymphedema surgery is becoming a highly promising approach to decrease the incidence of this debilitating condition.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction—The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-22-10/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-22-10/coif). The series “Breast Reconstruction—The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Ahmed RL, Prizment A, Lazovich D, et al. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol 2008;26:5689-96. [Crossref] [PubMed]
- Jørgensen MG, Toyserkani NM, Hansen FG, et al. The impact of lymphedema on health-related quality of life up to 10 years after breast cancer treatment. NPJ Breast Cancer 2021;7:70. [Crossref] [PubMed]
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. [Crossref] [PubMed]
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. Atlanta: American Cancer Society, 2017.
- Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol 2009;16:1959-72. [Crossref] [PubMed]
- Bartels SAL, Donker M, Poncet C, et al. Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981-22023 AMAROS Trial. J Clin Oncol 2023;41:2159-65. [Crossref] [PubMed]
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513-20. [Crossref] [PubMed]
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two andomized controlled trials. Lancet Oncol 2013;14:1086-94. [Crossref] [PubMed]
- Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, andomized, phase 3 trial. Lancet 2020;395:1613-26. [Crossref] [PubMed]
- Lasinski BB, McKillip Thrift K, Squire D, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R 2012;4:580-601. [Crossref] [PubMed]
- The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020;53:3-19. [PubMed]
- Scallan JP, Zawieja SD, Castorena-Gonzalez JA, et al. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 2016;594:5749-68. [Crossref] [PubMed]
- Brorson H, Ohlin K, Olsson G, et al. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphat Res Biol 2006;4:199-210. [Crossref] [PubMed]
- Koshima I, Kawada S, Moriguchi T, et al. Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg 1996;97:397-405; discussion 406-7. [Crossref] [PubMed]
- Yamamoto T, Yamamoto N, Yoshimatsu H, et al. Factors Associated with Lymphosclerosis: An Analysis on 962 Lymphatic Vessels. Plast Reconstr Surg 2017;140:734-41. [Crossref] [PubMed]
- Brorson H, Höijer P. Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J Plast Surg Hand Surg 2012;46:410-5. [Crossref] [PubMed]
- Ogata F, Narushima M, Mihara M, et al. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg 2007;59:180-4. [Crossref] [PubMed]
- Narushima M, Yamamoto T, Ogata F, et al. Indocyanine Green Lymphography Findings in Limb Lymphedema. J Reconstr Microsurg 2016;32:72-9. [PubMed]
- Yoshida RY, Kariya S, Ha-Kawa S, et al. Lymphoscintigraphy for Imaging of the Lymphatic Flow Disorders. Tech Vasc Interv Radiol 2016;19:273-6. [Crossref] [PubMed]
- Mitsumori LM, McDonald ES, Wilson GJ, et al. MR lymphangiography: How © do it. J Magn Reson Imaging 2015;42:1465-77. [Crossref] [PubMed]
- Neligan PC, Kung TA, Maki JH. MR lymphangiography in the treatment of lymphedema. J Surg Oncol 2017;115:18-22. [Crossref] [PubMed]
- Hayashi A, Giacalone G, Yamamoto T, et al. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast Reconstr Surg Glob Open 2019;7:e2086. [Crossref] [PubMed]
- Bianchi A, Visconti G, Hayashi A, et al. Ultra-High frequency ultrasound imaging of lymphatic channels correlates with their histological features: A step forward in lymphatic surgery. J Plast Reconstr Aesthet Surg 2020;73:1622-9. [Crossref] [PubMed]
- Chang DW, Masia J, Garza R 3rd, et al. Lymphedema: Surgical and Medical Therapy. Plast Reconstr Surg 2016;138:209S-18S. [Crossref] [PubMed]
- Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2013;132:1305-14. [Crossref] [PubMed]
- Pons G, Clavero JA, Alomar X, et al. Preoperative planning of lymphaticovenous anastomosis: The use of magnetic resonance lymphangiography as a complement to indocyanine green lymphography. J Plast Reconstr Aesthet Surg 2019;72:884-91. [Crossref] [PubMed]
- Becker C, Vasile JV, Levine JL, et al. Microlymphatic surgery for the treatment of iatrogenic lymphedema. Clin Plast Surg 2012;39:385-98. [Crossref] [PubMed]
- Ito R, Suami H. Overview of lymph node transfer for lymphedema treatment. Plast Reconstr Surg 2014;134:548-56. [Crossref] [PubMed]
- Miranda Garcés M, Pons G, Mirapeix R, et al. Intratissue lymphovenous communications in the mechanism of action of vascularized lymph node transfer. J Surg Oncol 2017;115:27-31. [Crossref] [PubMed]
- Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg 2012;255:468-73. [Crossref] [PubMed]
- Masia J, Pons G, Nardulli ML. Combined Surgical Treatment in Breast Cancer-Related Lymphedema. J Reconstr Microsurg 2016;32:16-27. [Crossref] [PubMed]
- Lee D, Piller N, Hoffner M, et al. Liposuction of Postmastectomy Arm Lymphedema Decreases the Incidence of Erysipelas. Lymphology 2016;49:85-92. [PubMed]
- Hoffner M, Ohlin K, Svensson B, et al. Liposuction Gives Complete Reduction of Arm Lymphedema following Breast Cancer Treatment-A 5-year Prospective Study in 105 Patients without Recurrence. Plast Reconstr Surg Glob Open 2018;6:e1912. [Crossref] [PubMed]
- Masià J, Pons G, Rodríguez-Bauzà E. Barcelona Lymphedema Algorithm for Surgical Treatment in Breast Cancer-Related Lymphedema. J Reconstr Microsurg 2016;32:329-35. [Crossref] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: a new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol 2009;16:703-8. [Crossref] [PubMed]
- Boccardo FM, Casabona F, Friedman D, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol 2011;18:2500-5. [Crossref] [PubMed]
- Yamamoto Y, Horiuchi K, Sasaki S, et al. Follow-up study of upper limb lymphedema patients treated by microsurgical lymphaticovenous implantation (MLVI) combined with compression therapy. Microsurgery 2003;23:21-6. [Crossref] [PubMed]
- Ishiura R, Yamamoto T, Saito T, et al. Comparison of Lymphovenous Shunt Methods in a Rat Model: Supermicrosurgical Lymphaticovenular Anastomosis versus Microsurgical Lymphaticovenous Implantation. Plast Reconstr Surg 2017;139:1407-13. [Crossref] [PubMed]
- Pons G, Condrea S, Masia J. Risk reducing lymphedema surgery: preliminary results with Targeted Lymphatic Axillary Repair (T-LAR) approach. 32nd EURAPS annual meeting, Naples, Italy. 2022.
Cite this article as: Pomata CD, Pons G, Masia J. Barcelona lymphedema algorithm for surgical treatment (BLAST). Ann Breast Surg 2024;8:7.