
Recent trends in nipple sparing mastectomy—a narrative review
Introduction
Great advances have been made in breast surgery over the past century. In 1882, Halsted performed the very first radical mastectomy with clearance of all breast tissue, the pectoralis major and associated lymphatic tissue (1). This was then modified by Patey in the 1940s to preserve the pectoral muscles. To date, the modified radical mastectomy (MSM) remains to be the conventional approach to breast removal in breast cancer patients with axillary nodal metastasis (2).
However, in recent years less aggressive measures are adopted for treatment of breast cancer patients. In the 1980’s, Fisher first introduced the concept of breast conservation therapy (BCT), which was followed by the development of skin-sparing and nipple sparing techniques in the late 1990’s (3). Notably, Hartmann’s paper published in 1999 demonstrated superiority of nipple sparing mastectomy (NSM) in high risk patients with a 90% reduction in breast cancer development (4). Since then, NSM has been performed frequently worldwide. We present this article in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-45/rc).
Methods
PubMed, EMBASE, CINAHL and Cochrane were searched for studies on NSM up till June 2020 (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 15th June 2020 |
| Databases and other sources searched | PubMed, EMBASE, CINAHL, Cochrane |
| Search terms used | Nipple sparing mastectomy; nipple areolar complex sparing mastectomy |
| Timeframe | Up until June 2020 |
| Inclusion criteria | English; any study type |
| Selection process | Literature review were conducted by both authors |
Literature review
Practices around the world
With the popularization of NSM, there have been multiple nation-wide studies highlighting its surgical and oncological outcome. More than 60 NSM series have been published since 2000, with the majority being major single center studies indicating acceptable rates of recurrence after NSM. To date, one of the largest studies was performed on 2,440 breast cancer patients receiving NSM between 1998 and 2013. This study showed 5- and 10-year DFS at 96.9% and 94.9% respectively, comparable to standard mastectomy techniques (5). In 2015, a study conducted on Italian populations showed that oncological outcomes were satisfactory with low rates of loco-regional recurrence (2.9%), NAC recurrence (0.7%), systemic recurrence (1%) and major surgical complications (4.4%) including NAC necrosis rate (around 4%) (6). There are some publications which review the application of NSM in Asian populations. A review conducted in Singapore shows that NSM is associated with a 5-year DFS of 90%, local recurrence rate of 4% and is therefore an oncologically safe procedure in selected patients with a relatively low complication rate (7). Another similar retrospective study was conducted in Hong Kong, which reflected a low recurrence rate of 4.1% and no delay of NAC excision from nipple necrosis (8). Overall, data published from specialised single centres show promising oncological outcome for NSM.
Indications for NSM
Although there is no definite consensus on which patients are selected for NSM, there are some guidelines in place for such candidates. The ultimate goal of NSM is to achieve negative resection margin (R0) without compromising cosmetic outcome. As such, a small tumour size of less than 3 cm, tumour at least 2 cm away from the areola-papillary complex (APC) and peripheral tumour are some of the suggested parameters for NSM (9). In addition to tumour size and location, tumours should be of low grade and not have any lymphovascular invasion, cutaneous involvement, axillary node metastasis, or human epidermal growth factor receptor-2 (HER-2) positivity (10). Essentially, all patients who are eligible for skin sparing mastectomy (SSM) could now be offered the alternative of NSM given that they fulfil the above criteria. Currently, patients with more advanced disease, large tumours or nodal-positive disease and prior radiation therapy may also be considered candidates for NSM (11-13). However, treatment modality should be individualized and depends on surgeon expertise and patient factors. In terms of patient factors, poor outcomes are associated with advanced age (>45 years old), active smoking, diabetes mellitus, hypertension, high body mass index (>30 kg/m2), previous radiation, macromastia (C cup or larger) and women with ptotic breasts (grade 2 or 3) (14,15). With the development of skin reduction techniques (16), simultaneous mastopexy and implant reconstruction (17), together with new incision techniques including a Wise pattern and bi-pedicle dermal flap (18), even ptotic breasts could undergo NSM. Recent studies have also shown some evidence to suggest that BRCA-carriers would benefit from prophylactic NSM (19).
Frontiers in NSM: staged breast reduction
Previously, macromastia and breast ptosis were contraindications for NSM due to the increased predilection for ischemic complications (20,21). The main purpose of staged breast reduction is to combat such complications. By reducing the skin envelope and repositioning the NAC, the rates of NAC and mastectomy flap necrosis are significantly reduced. In 2019, a matched cohort study was conducted between staged-breast reduction and non-staged techniques. This showed that in patients with a large breast size, staged breast reduction prior to NSM was associated with significantly lower rates of flap necrosis (22). A newly published retrospective study in 2020 performed bilateral reduction and mastopexy followed by NSM with immediate reconstruction using free abdominal flaps on breast cancer or high-risk BRCA patients. Those who have undergone such an algorithm had clear margins in all cases, with minimal complications including NAC necrosis (partial 8.2% vs. complete 6.6%) and no flap loss in any of the cases (23). As such, staged breast reduction as a surgical technique is indicated in large and ptotic breasts (24). In addition, the timing of pre-mastectomy reduction is crucial. When performed in the same setting as a single-staged procedure, the vasculature to the skin would be disturbed, increasing rates of complications (25). Therefore, in general practice, NSM is generally planned for 3 months after reduction, with an average period of 5 months between stages. Some studies report a minimum of 4 weeks between stages, which give the additional benefit of having smaller, nodal-negative tumours or in situ carcinomas (26).
Techniques of NSM
Vasculature of the breast
The nipple areolar complex (NAC) is mainly supplied by vasculature stemming from the subclavian artery. Specifically, the main arterial supply to the medial aspect of the breast is via the perforating branches of the internal thoracic artery. Branches from the lateral thoracic and posterior intercostal arteries also provide blood supply to the breast (27). Instead of following the ductal system, these vessels follow a path between the skin and the breast parenchyma. In NSM, blood supply preservation is of a high priority as nipple necrosis is a well-documented complication (28). Anatomical variation of vascular supply is frequently documented. Although most cases of NAC receive principal supply from the second intercostal perforator originating from the internal mammary artery, dual supply is also evident in some cases. A study conducted by Bahl reported considerably lower necrosis rates in dual-supply breasts, hence a lesser need for debridement. In the future, pre-operative MRI could be useful in the delineation of breast vascularity, and in the subsequent surgical planning of NSM (28,29).
Different incisions for the NSM
There are numerous variations for incisions in NSM. Amongst these incisions, the four common ones are the peri-areolar, lateral, inframammary and vertical incisions (15). The characteristics of the different incisions are summarized in the Table 2.
Table 2
| Incisions | Description | Advantage | Disadvantage | Risks of NAC necrosis | Target patient group |
|---|---|---|---|---|---|
Periareolar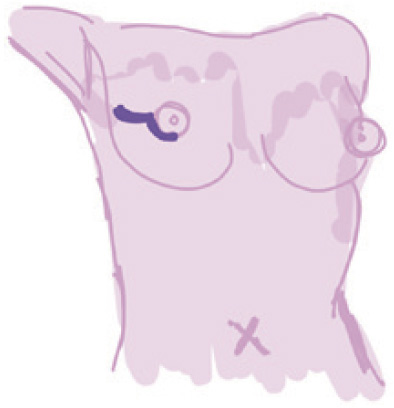 |
Curvilinear incision along areola | (I) Well concealed scar; (II) adequate access to all breast quadrants; (III) good access for sentinel lymph node and axillary dissections | (I) Technical difficulty in performing; (II) low nipple areola complex sensitivity after surgery | Highest (18.1%) (disruption to the dermal vascular plexus) | Larger breasts or higher grade ptosis |
Vertical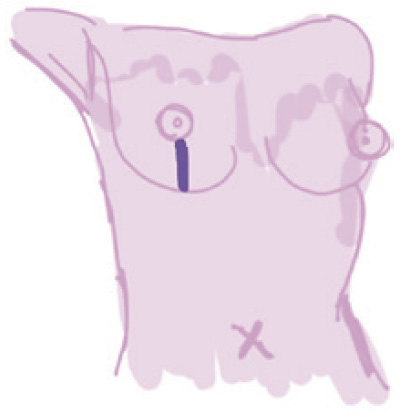 |
Linear incision from NAC directed inferiorly | (I) Ease of dissection; (II) precise inset; (III) setup for revision | (I) Higher wound healing issues in comparison to a mid-breast incision; (II) incision at least 6 cm to accommodate flap inset | Medium (incision lies between and runs parallel to medial/lateral mammary arteries) | Non-ptotic breasts Small breasts |
Lateral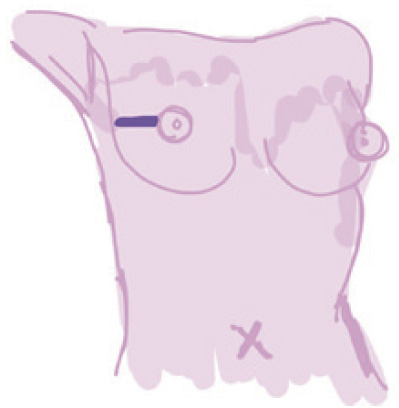 |
Linear incision from the lateral border of breast toward NAC | (I) Easy access to all quadrants of breast tissue, sentinel lymph node biopsy and axillary tail; (II) autologous reconstruction | (I) Obvious scar; (II) contraction results in nipple malposition; (III) flattening of the breast, particular after large tissue removal | Relatively low (less disruption to the subdermal vascular supply) | – |
Inframammary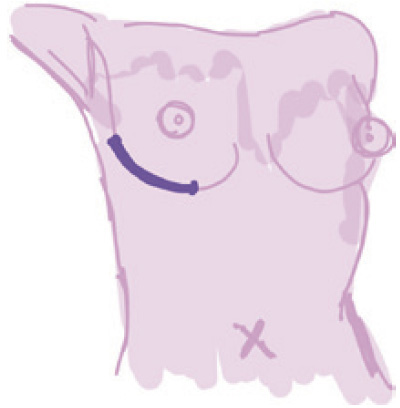 |
Incision along the inframammary fold | (I) Well concealed scar; (II) excellent visualization and palpation of the underlying breast gland | Difficulty in accessing breast tissue in upper quadrants | Lowest (6.82%) (spares internal thoracic artery supply, may disrupt the hypervascular zone ant to the inf border of the pectoralis major) | Small breasted women |
NAC, nipple areolar complex.
Of note, the transareolar incision, which involves an incision bivalving the nipple from the medial and lateral sides, has yielded an exceptionally high NAC necrosis rate exceeding 80% (23). It has therefore become obsolete in recent times. According to a meta-analysis performed in 2019 (n=9,975), the inframammary approach has become the most popular incision, largely due to its acceptable cosmesis, suitability for single-stage reconstruction and low rate of NAC necrosis. In a recently conducted study, the inframammary fold NSM (IMF NSM) was shown to accommodate larger implants in relation to weight of the breast when compared to nipple sacrificing mastectomy in implant reconstruction with biological mesh. In comparison to the nipple sacrificing mastectomy group, the IMF NSM patients had no incidence of wound necrosis or threatened wound (30). However, not all cases are suitable for inframammary incisions, and the decision on the type of incision is still a multi-disciplinary opinion.
Novel approaches of the NSM include the robotic technique, which has limited data reflecting long-term outcome and remains to be in the assessment phase (31). Careful attention must be paid when selecting the method of incision as it also impacts on clinical outcomes, medical cost and aesthetic result. In a recently performed case-control study in 2020, the surgical margin involvement rate in both robotic-NSM (2.5%) and endoscopic-NSM (4.44%) were relatively low (P=0.52). The robotic-NSM showed superiority in satisfaction rate, cosmetic outcome, blood loss and surgical wound position in comparison to the endoscopic-NSM group (32). However, robotic-NSM was also associated with a longer operation duration and higher medical cost (10,587+554 vs. 6,855+936 US dollars). According to a meta-analysis performed in 2019, endoscopic incisions have the lowest rates of NAC necrosis at 4.9% (33).
Oncologic safety of NSM—therapeutic NSM
As of the time of this publication, there are no published randomised controlled trials (RCTs) of NSM, with limited long term follow up in clinical series. There have, however, been multiple systematic reviews and meta-analysis performed on the oncological safety of NSM. In 2018, a systematic review was conducted which showed no difference between NSM and SSM in 5-year disease-free survival (DFS) and mortality rates. Similarly, local recurrence rates were acceptably low at 3.9% and 3.3% respectively (24). A further meta-analysis performed in 2015 showed that local recurrence and survival rates in NSM were comparable to MRM and SSM (25). In a systematic review published in 2019, BCT was shown to be a feasible alternative to mastectomy in BRCA carriers owing to the non-inferior survival outcomes despite increase in local recurrence rate (33). These results are promising and institutions are now expanding NSM to include more patient groups. The low levels of recurrence and high rates of DFS are also echoed in more recent studies, with Table 3 summarizing the relevant data in NSM (13,33-37).
Table 3
| Study | Study type (size) | Follow-up time (months) | Overall survival (%) | DFS | Local recurrence (%) |
|---|---|---|---|---|---|
| 2020, Parvez (33) | Cohort (n=175) | 24 | 98.3 | 88.6% | 4.6 |
| 2019, Frey (34) | Retrospective (n=496) | 48 | N/A | N/A | 1.6 |
| 2019, Balci (35) | Retrospective (n=214) | 62 | N/A | DFS: 93.2% (TND <2 cm), 96.3% (TND ≥2 cm) at 10 years | 4.7 |
| 2018, Li (36) | Retrospective (n=118) | 53 | 92.4 | DFS 91.5% at 3 years | 3.4 |
| 2018, Lago (37) | Retrospective (n=69) | 143 | 98.5 | 88.4% | 11.6 |
| 2017, Smith (13) | Cohort (n=2,182) | 51 | N/A | 95.7% at 3 years and 92.3% at 5 years | 3.7 |
NSM, nipple sparing mastectomy; DFS, disease-free survival; N/A, not available; TND, tumor to nipple distance.
At first glance, the results seem promising with low local recurrence and high DFS. However, the study conducted by Lago in 2018 showed that local recurrence is significantly higher. This could be attributable to the significantly longer follow-up time, even with a small sample size of 69 patients. The argument against NSM is formed on the basis that there is occult malignancy with NAC involvement. In fact, previous studies have indicated that there is NAC involvement in up to 58% (32) of NSM patients, with the overall rate at 11.5% (6). This is attributable to ductal and lobular cancers with terminal ductal lobular units (TDLUs). Nonetheless, there is strong evidence to suggest that NSM is associated with a low recurrence rate in carefully selected cases of sporadic breast cancer (8).
Applications of NSM in BRCA mutation carriers—prophylactic NSM
Cosmetic outcome may be particularly of concern as women with BRCA mutations requiring mastectomy belong to a younger demographic. However, BRCA mutation carriers are also at a higher predisposition of developing more aggressive disease (8), and oncological outcome should not be sacrificed for cosmesis. There is a role for prophylactic NSM in BRCA mutation carriers, as NSM offers superior cosmetic outcome. Previously, this role was small as there were limited studies published and published data do not have large sample sizes. In recent years, there has been an increase in popularity for NSM in BRCA carrier patients, with an increase in the relevant data sets. Recent data all demonstrated that no ipsilateral breast cancers occurred after prophylactic NSM in BRCA carriers (9,38).
Counselling of the patient for NSM
NSM offers superior cosmetic outcome and enhanced post-mastectomy body image. This has a positive impact on psychosocial well-being and self-esteem. However, the patient should be properly counselled prior to NSM and warned of the possible complications which may arise. Rates of skin necrosis could be up to 8%, and skin desquamation is not uncommon accounting for around 40% of cases. There is also a possibility for re-operation to deal with post-operative complications (10%), including implant removal (3.4%). There is also a 5% possibility that nipple excision may be required, in cases where cancer is found at the nipple margin. Other complications include hematoma (3%), seroma and infection (6%) (38,39). This percentage varies between studies and surgeon experience, so the local statistic should be used for counselling of the patient in NSM (40).
Conclusions
NSM offers better cosmetic outcomes and non-inferior survival outcomes, in carefully selected patient groups with sporadic breast cancer and in BRCA mutation carriers. Further studies would be useful in elucidating the long-term sequelae of NSM in both BRCA carriers and sporadic breast cancer patient groups.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Masakazu Toi) for the series “Cutting-edge Surgical Research” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-45/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-45/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-45/coif). The series “Cuting-edge Surgical Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shay P, Jacobs J. Autologous reconstruction following nipple sparing mastectomy: a comprehensive review of the current literature. Gland Surg 2018;7:316-24. [Crossref] [PubMed]
- Lanitis S, Hadjiminas DJ. From radical to nipple sparing mastectomy: Techniques, indications and safety. Hellenic Journal of Surgery 2015;87:215-23. [Crossref]
- Ashikari AY, Kelemen PR, Tastan B, et al. Nipple sparing mastectomy techniques: a literature review and an inframammary technique. Gland Surg 2018;7:273-87. [Crossref] [PubMed]
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999;340:77-84. [Crossref] [PubMed]
- Weber WP, Haug M, Kurzeder C, et al. Oncoplastic Breast Consortium consensus conference on nipple-sparing mastectomy. Breast Cancer Res Treat 2018;172:523-37. [Crossref] [PubMed]
- Orzalesi L, Casella D, Santi C, et al. Nipple sparing mastectomy: Surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast 2016;25:75-81. [Crossref] [PubMed]
- Ng YY, Tan VK, Pek WS, et al. Surgical and oncological safety of nipple-sparing mastectomy in an Asian population. Breast Cancer 2019;26:165-71. [Crossref] [PubMed]
- Chan YH, Yau WM, Cheung PS. Oncological Safety and Technical Feasibility of Nipple-Sparing Mastectomy for Breast Cancer: The Hong Kong Experience. World J Surg 2018;42:1375-83. [Crossref] [PubMed]
- Pires D, Fonseca E, Valadares C, et al. Nipple sparing mastectomy: a literature review. Mastology 2018;28:40-5. [Crossref]
- Mallon P, Feron JG, Couturaud B, et al. The role of nipple-sparing mastectomy in breast cancer: a comprehensive review of the literature. Plast Reconstr Surg 2013;131:969-84. [Crossref] [PubMed]
- Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol 2013;20:3218-22. [Crossref] [PubMed]
- Krajewski AC, Boughey JC, Degnim AC, et al. Expanded Indications and Improved Outcomes for Nipple-Sparing Mastectomy Over Time. Ann Surg Oncol 2015;22:3317-23. [Crossref] [PubMed]
- Smith BL, Tang R, Rai U, et al. Oncologic Safety of Nipple-Sparing Mastectomy in Women with Breast Cancer. J Am Coll Surg 2017;225:361-5. [Crossref] [PubMed]
- Tousimis E, Haslinger M. Overview of indications for nipple sparing mastectomy. Gland Surg 2018;7:288-300. [Crossref] [PubMed]
- Co M, Chiu R, Chiu TM, et al. Nipple-Sparing Mastectomy and Its Application on BRCA Gene Mutation Carrier. Clin Breast Cancer 2017;17:581-4. [Crossref] [PubMed]
- Dietz J, Fedele G. Skin Reduction Nipple-Sparing Mastectomy. Ann Surg Oncol 2015;22:3404. [Crossref] [PubMed]
- Folli S, Mingozzi M, Curcio A, et al. Nipple-sparing mastectomy: an alternative technique for large ptotic breasts. J Am Coll Surg 2015;220:e65-9. [Crossref] [PubMed]
- Martinovic ME, Pellicane JV, Blanchet NP. Surgical Delay of the Nipple-Areolar Complex in High-risk Nipple-sparing Mastectomy Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e760. [Crossref] [PubMed]
- Jakub JW, Peled AW, Gray RJ, et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population With BRCA Mutations: A Multi-institutional Study. JAMA Surg 2018;153:123-9. [Crossref] [PubMed]
- Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg 2006;118:832-9. [Crossref] [PubMed]
- Wang F, Alvarado M, Ewing C, et al. The impact of breast mass on outcomes of total skin-sparing mastectomy and immediate tissue expander-based breast reconstruction. Plast Reconstr Surg 2015;135:672-9. [Crossref] [PubMed]
- Salibian AA, Frey JD, Karp NS, et al. Does Staged Breast Reduction before Nipple-Sparing Mastectomy Decrease Complications? A Matched Cohort Study between Staged and Nonstaged Techniques. Plast Reconstr Surg 2019;144:1023-32. [Crossref] [PubMed]
- Momeni A, Kanchwala S, Sbitany H. Oncoplastic Procedures in Preparation for Nipple-Sparing Mastectomy and Autologous Breast Reconstruction: Controlling the Breast Envelope. Plast Reconstr Surg 2020;145:914-20. [Crossref] [PubMed]
- Yazar S, Bengur FB, Altinkaya A, et al. Nipple-Sparing Mastectomy and Immediate Implant-Based Reconstruction with or Without Skin Reduction in Patients with Large Ptotic Breasts: A Case-Matched Analysis. Aesthetic Plast Surg 2021;45:956-67. [Crossref] [PubMed]
- Spear SL, Rottman SJ, Seiboth LA, et al. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg 2012;129:572-81. [Crossref] [PubMed]
- van Deventer PV. The blood supply to the nipple-areola complex of the human mammary gland. Aesthetic Plast Surg 2004;28:393-8. [Crossref] [PubMed]
- Stolier A. Techniques to Avoid Nipple and Flap Necrosis. 2017. p. 101-15.
- Bahl M, Pien IJ, Buretta KJ, et al. Can Vascular Patterns on Preoperative Magnetic Resonance Imaging Help Predict Skin Necrosis after Nipple-Sparing Mastectomy? J Am Coll Surg 2016;223:279-85. [Crossref] [PubMed]
- Mathew J. Can we safely accommodate larger volume implants in inframammary fold nipple sparing mastectomy compared to nipple sacrificing mastectomy in implant-based reconstruction with acellular dermal matrix? JPRAS Open 2021;27:1-6. [Crossref] [PubMed]
- Angarita FA, Castelo M, Englesakis M, et al. Robot-assisted nipple-sparing mastectomy: systematic review. Br J Surg 2020;107:1580-94. [PubMed]
- Lai HW, Chen ST, Tai CM, et al. Robotic- Versus Endoscopic-Assisted Nipple-Sparing Mastectomy with Immediate Prosthesis Breast Reconstruction in the Management of Breast Cancer: A Case-Control Comparison Study with Analysis of Clinical Outcomes, Learning Curve, Patient-Reported Aesthetic Results, and Medical Cost. Ann Surg Oncol 2020;27:2255-68. [Crossref] [PubMed]
- Daar DA, Abdou SA, Rosario L, et al. Is There a Preferred Incision Location for Nipple-Sparing Mastectomy? A Systematic Review and Meta-Analysis. Plast Reconstr Surg 2019;143:906e-19e. [Crossref] [PubMed]
- Parvez E, Martel K, Morency D, et al. Surgical and Oncologic Outcomes of Nipple-Sparing Mastectomy for a Cohort of Breast Cancer Patients, Including Cases with High-Risk Features. Clin Breast Cancer 2020;20:353-8. [Crossref] [PubMed]
- Frey JD, Salibian AA, Lee J, et al. Oncologic Trends, Outcomes, and Risk Factors for Locoregional Recurrence: An Analysis of Tumor-to-Nipple Distance and Critical Factors in Therapeutic Nipple-Sparing Mastectomy. Plast Reconstr Surg 2019;143:1575-85. [Crossref] [PubMed]
- Balci FL, Kara H, Dulgeroglu O, et al. Oncologic safety of nipple-sparing mastectomy in patients with short tumor-nipple distance. Breast J 2019;25:612-8. [Crossref] [PubMed]
- Li ZJ, Zhang P, Zhang W, et al. Oncological safety and prognosis factors analysis of immediate breast reconstruction after nipple-areola-complex sparing mastectomy. Zhonghua Zhong Liu Za Zhi 2018;40:690-5. [PubMed]
- Lago V, Maisto V, Gimenez-Climent J, et al. Nipple-sparing mastectomy as treatment for patients with ductal carcinoma in situ: A 10-year follow-up study. Breast J 2018;24:298-303. [Crossref] [PubMed]
- Carlson GW, Chu CK, Moyer HR, et al. Predictors of nipple ischemia after nipple sparing mastectomy. Breast J 2014;20:69-73. [Crossref] [PubMed]
- Valero MG, Muhsen S, Moo TA, et al. Increase in Utilization of Nipple-Sparing Mastectomy for Breast Cancer: Indications, Complications, and Oncologic Outcomes. Ann Surg Oncol 2020;27:344-51. [Crossref] [PubMed]
- Wu ZY, Kim HJ, Lee JW, et al. Oncologic Outcomes of Nipple-sparing Mastectomy and Immediate Reconstruction After Neoadjuvant Chemotherapy for Breast Cancer. Ann Surg 2021;274:e1196-201. [Crossref] [PubMed]
Cite this article as: Cheung C, Co M. Recent trends in nipple sparing mastectomy—a narrative review. Ann Breast Surg 2024;8:8.

