Immediate nipple areolar complex reconstruction over latissimus dorsi skin paddle flap in bilateral periareolar mastectomy & reconstruction
Introduction
Oncoplastic surgery has been widely developed during the last decades. Oncological security has been assured in these procedures. Skin sparing mastectomy (SSM) is widely accepted when the nipple-areola complex (NAC) cannot be preserved (1). Different types of reconstruction can be addressed in these cases as distant or local autologous tissue, or expanders-implant devices. When the skin envelop is not completely reliable, an additional improvement is recommended. In our Institution we have increasingly used the latissimus dorsi (LD) flap with the modification of NAC reconstruction over the skin paddle. The breast envelop is returned to its original dimensions, the NAC is reconstructed and the envelop quality regarding thickness is now properly adjusted for safely covering the expander or implant with the muscle (2).
Based on technical modifications the latissimus dorsi flap (LDF) has intrinsic features that provides a wide range of surgical options to achieve satisfactory aesthetic result (3).
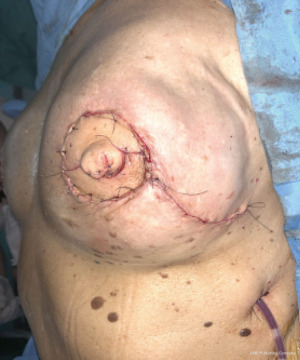
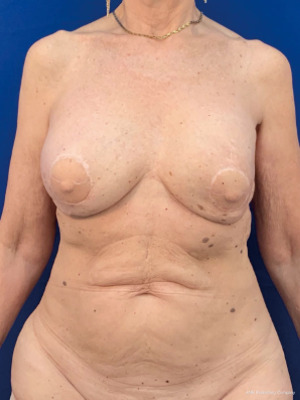
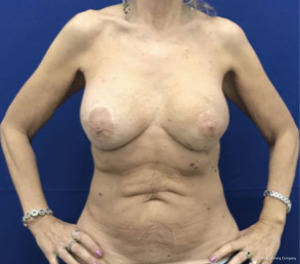
The oncoplastic surgical process has a final step, the creation of the NAC. It used to be considered as a secondary complement to breast reconstruction. Nowadays, it is a key element, not only for the psychological impact in women, but also because marks the end of the medical process. NAC is usually reconstructed after an interval of several months and it could be done by different techniques such as local flaps or composite graft of the opposite NAC. The authors performed an immediate NAC reconstruction with a local flap over the LD paddle, acquiring satisfactory outcomes in relation to symmetry, position, size, shape, texture, color, and long-term projection (Figure 1).
The purpose of this study is to present a combination of techniques that allows for treating breast cancers in bilateral skin sparing mastectomies with an immediate reconstruction using LDFs. This offers versatility as it contributes volume to the breast and adds the possibility of reconstructing the entire breast envelope with the NAC on its skin paddle in a single surgery. We present the following article in accordance with the STROBE reporting checklist (https://abs.amegroups.com/article/view/10.21037/abs-21-144/rc).
Methods
A retrospective review was carried out of 12 patients who had undergone immediate breast and nipple reconstruction with bilateral LDF after SSM, in a single surgical time. The period in which these surgeries were performed was between January 2013 and June 2019. Mean follow-up time was 32.2 months.
Statistical analysis
Patient characteristics and statistical analyses are reported in Table 1.
Table 1
| Patient | Age (years) | Disease | Type of SSM | Implant/expander | Implant characteristics | Hospitalization (days) | Complications | Follow up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 53 | Bilateral breast cancer w/nipple secretion | Periareolar SSM | Anatomical expander 345 cc | Round 300 cc | 2 | No | 12 |
| 2 | 62 | BRACA w/periareolar scars | Periareolar SSM | Anatomical expander 300 cc | Anat. 295 cc | 1 | No | 72 |
| 3 | 49 | Bilateral Br Ca nipple involvement | Periareolar SSM | Anatomical expander 300 cc | Round 400 cc | 1 | Seroma | 6 |
| 4 | 64 | BRACA w/previous mastopexy | Periareolar SSM | Anatomical expander 400 cc | Anat. 345 cc | 1 | Seroma | 48 |
| 5 | 55 | Br Ca with previous mastopexy | Periareolar SSM | Anatomical expander 345 cc | Round 400 cc | 1 | Seroma/dehiscence | 24 |
| 6 | 47 | BRACA w/previous mastopexy | Periareolar SSM | Anatomical expander 400 cc | Anat. 255 cc | 2 | Seroma | 6 |
| 7 | 38 | Bilateral breast cancer w/nipple retraction | Periareolar SSM | Anatomical expander 255 cc | Anat. 2955 cc | 1 | No | 36 |
| 8 | 60 | BRACA w/previous mastopexy | Periareolar SSM | Anatomical expander 345 cc | Anat. 295 cc | 2 | Seroma | 52 |
| 9 | 58 | BRACA w/previous mastopexy | Periareolar SSM | Anatomical expander 295 cc | Anat. 345 cc | 1 | No | 48 |
| 10 | 46 | Bilateral breast cancer with periareolar scars | Periareolar SSM | Anatomical expander 345 cc | Anat. 345 cc | 1 | No | 23 |
| 11 | 71 | BRACA w/periareolar scars | Periareolar SSM | Anatomical expander 350 cc | Round 350 cc | 2 | Seroma | 36 |
| 12 | 42 | BRACA w/previous mastopexy | Periareolar SSM | Anatomical expander 345 cc | Anat. 345 cc | 2 | No | 24 |
SSM, skin sparing mastectomy; w, with; Br Ca, breast cancer; Anat., anatomical.
Inclusion criteria: women, no ptosis, BRCA (+) or breast cancer, no viability of NAC after mastectomy.
Exclusion criteria: breast ptosis, under 18 years old, comorbidities.
The study was approved by the Institutional Review Board of Instituto Oncológico Henry Moore University of Buenos Aires (IRB No. HM-12008) and performed in accordance with the principles of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all individual participants.
Results
In this limited series of 12 patients (24 procedures), the mean age was 57 years (range, 38–71 years); patients were selected with no comorbidities and no overweight. As complications, seroma at the dorso was the most common 5/12 (40%); and bilateral minimal back wound dehiscence in the same patient with spontaneous good evolution. No reinterventions were needed. Success in NAC reconstruction was 100%.
Operative technique
Surgical position
Ventral position with arms abducted at 90 degrees to perform the SSM and transfer and closure of the LDF.
Dorsal position with arms abducted at 90 degrees to perform LDF.
Marking
Breast markings
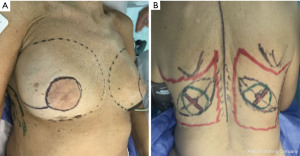
SSM marking must be designed depending on the absence or presence of scars biopsies and breast contouring (ptosis and excess volume). Usually done as a periareolar mark with extension to scars, biopsies, axillar dissections (Figure 2A) or an inverted T pattern, when volume excess is present and mastopexy is associated (4).
Back markings
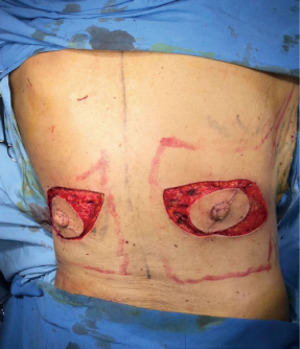
The limits of the entire LD muscle are marked according to the anatomical concerns such as scapula tip superiorly, vertebral origins medially (from T7 down). Over the upper portion of the muscle, we designed the skin paddle in an oblique, “banana” design, that could vary in position (medial to lateral) and dimensions (up to 10 × 30 cm) depending on breast requirements (Figure 2B).
Markings over the breast are performed around the NAC with a lateral curved incision up to the external breast footprint. Thickness of the flaps must be individually evaluated previously with MRI and mammography seeing fat coverage. In addition, surgical dissection must be meticulous and uniform across the Cooper ligaments to provide oncological security and preservation of flap vitality (5).
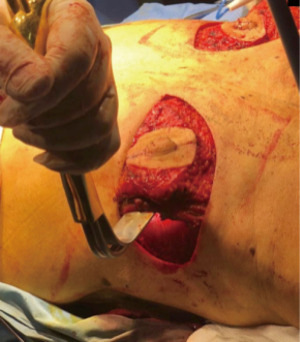
NAC designed on skin paddle
The preferred NAC reconstruction technique for the authors is the C-V flap. Designed over the skin paddle island on the LDF. Performed before the LDF are dissected (Figure 3). It consists in two lateral V flaps and one central C flap; the V flap are elevated subcutaneously, and donor site closed primarily. The C flap is elevated as dermal flap where both V flaps wrapped around, and the C flap are placed on top of them as a cap. The rest of skin that is not used for C-V flap is de-epithelized and preserved to increase periareolar breast volume. NAC reconstruction over the LD skin paddle maintains the projection over the time thanks to the LD dermis thickness, loosing less than 25% after two years. Success rate for NAC reconstruction was 100%.
LDF dissection technique
The incision proceeds around the skin island to the subcutaneous tissue, increasing subcutaneous volume to incorporate perforator vessels. A layer of fat is preserved over the LD by tailoring flap dissection at the thoracic fascia. This allows preservation of perforator vessels to maintain NAC vitality. The fatty layer Incorporation into the overall volume provided by the flap is an important element to make maximal profit of the LD musculocutaneous flap (6-8).
Dissection extends onto the limits of the LD muscle (Figure 4).
The thoracodorsal pedicle enters the deep plane of the LD 8 to 10 cm below the axillary vessels and 2.5 to 3 cm from lateral margins. Dissection proceeds along 2–3 cm above the entrance of the pedicle. Distal musculotendinous insertion (in the proximal humerus) is released to increase the arch of rotation and for better recreation of the axillary fold. Care is taken to avoid tension on the vascular pedicle which is now completely liberated. Before muscle transfer, the thoracodorsal nerve is cut to avoid animation under loop magnification (9).
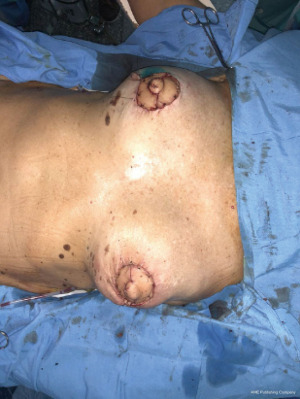
Flap viability is essential and must be checked before transfer. A subcutaneous tunnel is developed high in the axilla, the flap is transferred first to the axilla and then to the breast. At this point the flap length is checked to see if it will reach the areas to be restore specially the periareolar defect, and any necessary revision are made at this time. Drains are placed for the back and for expander pockets (10).
For final results, depending on reconstructive requirement, different surgical options might be applied to the procedure such as breast expanders, implants or fat grafting (Figures 5,6).
Discussion
Conservative mastectomies incorporate the benefit of tumor and total gland excision as traditionally done by radical mastectomies, preserving breast envelope and NAC, if possible. For those cases where NAC must be sacrificed, the SSM allows the preservation of original NAC position, determine by the rest of the breast envelope conserved (11).
Significant advances in surgical techniques had been incorporated over the last 30 years in reconstructive breast surgery. Each of the major techniques includes the use of implants with or without tissue expanders, the LDF, the transverse rectus abdominis musculocutaneous (TRAM) flap, various free flaps. All the reconstructive options had a gold period; we have used the LDF, as a consistent option for cases where bilateral mastectomy was unavoidable, and NAC was not possible to preserve. The LDF incorporates volume not only because on the muscle but also on the fat layer superficial to the LD fascia; that increments around 300 cc in a patch of 20 cm × 30 cm flap. Moreover, it is possible to increase volume using tissue expander or implants, achieving high specific volumes, making better matches between both breasts. Also, fat transfer is other way to increase volume and mitigate discrepancies between both breasts without the need to use implants (12). Comparably to other autologous flaps like TRAM, or free flaps from abdomen, where muscle harvest implicate functional morbidity as muscle wall weakness, usually high scarring mal positions with unpleasant aesthetic results. The LDF has a donor site scar placed on the back over the relax skin tension lines, resulting in a fine, smooth, and less visible scar (13). Finally, LDF skin island has a thick dermis, more than any other flap technique for NAC reconstruction; this allows a more consistent and long-lasting result as compared with skin quality of TRAM flap or the post expanded breast skin. Moreover, skin dorsum has a different color compared to breast skin and tattooing might not be necessary. The technique used is a C-V flap deigned over an oblique back pattern in order to place the remaining scar over the skin tension lines of the back (14,15). This C-V flap is tailored in the ventral decubitus position before the transfer of the LDF to the breast, as the tissue is more stable, and the thick dermis of the dorsum makes the flap confection technically easier and lowers operative time with minimal changes in outcomes, complications, or patient satisfaction (16,17) (Figure 7).
Complications
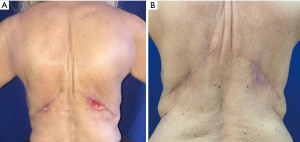
The major complication resulting from elevation of the LD musculocutaneous flap is the formation of a persistent back seroma. Sometimes, the back drain may remain for as long as 6 weeks postoperatively. Any recurrent fluid accumulation will be treated with outpatient percutaneous aspiration. Rarely, a scar capsule may develop in the back and may necessitate surgical removal and drainage. Two small superficial incision dehiscence are reported (Figure 8). Loosing of the muscle functionality is usually well tolerated, with only the most athletic patients noting any function loss.
Limitations to this study
Results with this approach with this small series with careful patient selection is a limitation to our conclusions, and a larger number of patients is needed to confirm our results.
Conclusions
For those cases where bilateral SSM must be associated with NAC resection, Bilateral LDF reconstruction with NAC tailoring over the LD skin paddle is a remarkable reconstructive option.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nicola Rocco, Giacomo Montagna and Giuseppe Catanuto) for the series “New Perspectives in Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-144/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-144/dss
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-144/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-144/coif). The series “New Perspectives in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Instituto Oncológico Henry Moore University of Buenos Aires (IRB No. HM-12008) and performed in accordance with the principles of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rancati A, Gercovich FG. Introduction to conservative mastectomies. Gland Surg 2015;4:450-2. [PubMed]
- Schneider WJ, Hill HL Jr, Brown RG. Latissimus dorsi myocutaneous flap for breast reconstruction. Br J Plast Surg 1977;30:277-81. [Crossref] [PubMed]
- Olivari N. The latissimus flap. Br J Plast Surg 1976;29:126-8. [Crossref] [PubMed]
- Hammond DC. Latissimus dorsi flap breast reconstruction. Clin Plast Surg 2007;34:75-82; abstract vi-vii. [Crossref] [PubMed]
- McCraw JB, Papp C, Edwards A, et al. The autogenous latissimus breast reconstruction. Clin Plast Surg 1994;21:279-88. [Crossref] [PubMed]
- Delay E, Gounot N, Bouillot A, et al. Autologous latissimus breast reconstruction: a 3-year clinical experience with 100 patients. Plast Reconstr Surg 1998;102:1461-78. [Crossref] [PubMed]
- Papp C, McCraw JB. Autogenous latissimus breast reconstruction. Clin Plast Surg 1998;25:261-6. [Crossref] [PubMed]
- Rose EH, Vistnes LM, Ksander GA. The panniculus carnosus in the domestic pig. Plast Reconstr Surg 1977;59:94-7. [Crossref] [PubMed]
- Besana-Ciani I, Greenall MJ. Langer's axillary arch: anatomy, embryological features and surgical implications. Surgeon 2005;3:325-7. [Crossref] [PubMed]
- Halperin TJ, Fox SE, Caterson SA, et al. Delayed division of the thoracodorsal nerve: a useful adjunct in breast reconstruction. Ann Plast Surg 2007;59:23-5. [Crossref] [PubMed]
- Delay E, Mojallal A, Vasseur C, et al. Immediate nipple reconstruction during immediate autologous latissimus breast reconstruction. Plast Reconstr Surg 2006;118:1303-12. [Crossref] [PubMed]
- Fisher J, Hammond DC. The combination of expanders with autogenous tissue in breast reconstruction. Clin Plast Surg 1994;21:309-20. [Crossref] [PubMed]
- Sternberg EG, Perdikis G, McLaughlin SA, et al. Latissimus dorsi flap remains an excellent choice for breast reconstruction. Ann Plast Surg 2006;56:31-5. [Crossref] [PubMed]
- Mimoun M, Chaouat M, Lalanne B, et al. Latissimus dorsi muscle flap and tissue expansion for breast reconstruction. Ann Plast Surg 2006;57:597-601. [Crossref] [PubMed]
- Laitung JK, Peck F. Shoulder function following the loss of the latissimus dorsi muscle. Br J Plast Surg 1985;38:375-9. [Crossref] [PubMed]
- Fraulin FO, Louie G, Zorrilla L, et al. Functional evaluation of the shoulder following latissimus dorsi muscle transfer. Ann Plast Surg 1995;35:349-55. [Crossref] [PubMed]
- Nahabedian MY. Achieving ideal breast aesthetics with autologous reconstruction. Gland Surg 2015;4:134-44. [PubMed]
Cite this article as: Rancati A, Angrigiani C, Dorr J, Cortiñas M, Rancati A, Acquaviva J, Bou M. Immediate nipple areolar complex reconstruction over latissimus dorsi skin paddle flap in bilateral periareolar mastectomy & reconstruction. Ann Breast Surg 2023;7:36.