The development of autologous breast reconstruction and the impact of enhanced recovery after surgery (ERAS): a narrative review
Introduction
Functional, social, and psychological rehabilitation are an essential part of breast cancer treatment. Even with breast-conserving surgery as an available alternative, many women with breast cancer still undergo a mastectomy.
More than 80% of the women so treated show interest in breast reconstruction after the initial treatment (1), and, with a 5-year survival rate of more than 85% (2), it has become an integrated part of breast cancer treatment. As the incidence of breast cancer is growing and the use of radiotherapy limits implant-based reconstruction, the demand for reconstructions using autologous tissue has increased.
Autologous breast reconstruction (ABR) can be performed in both irradiated and nonirradiated patients. However, patients who have undergone radiotherapy should ideally have autologous reconstruction, as complication rates in irradiated patients are unacceptably high (3).
Either or both breasts can be reconstructed in the same procedure, and the reconstruction can be performed either at the time of the mastectomy or as a delayed procedure. The goal of the surgery is to remove the bothersome external prosthesis and, more importantly, to provide women with the feeling of wholeness, thus helping to alleviate the physiological and psychological trauma related to breast cancer (4). The importance of ABR for psychological well-being is well-documented, but whether it can offer additional benefits for pain, lymphedema, and other complaints is still being investigated.
The deep inferior epigastric perforator (DIEP) flap has been the gold standard in ABR for well over a decade. This perforator-based flap from the abdomen delivers the best possible tissue, allows for excellent shaping, and has very low complication rates. Flap survival is typically reported to be above 98%, and the treatment is well established worldwide (5-7). The musculocutaneous latissimus dorsi (LD) flap with a permanent implant is another workhorse in reconstructive plastic surgery. In our unit, it is a commonly used, safe, and viable alternative to the DIEP flap or other free flaps.
Enhanced recovery after surgery (ERAS), or fast-track surgery, was described almost 20 years ago as a peri- and postoperative care concept with the aim of achieving a pain- and risk-free operation (8). Since then, many surgical specialties have embraced the concept, and it is widely accepted that enhanced recovery programs (ERPs) can be superior to conventional care for a wide range of surgical procedures, including microsurgical reconstruction, and can provide substantial economic benefits (9-14). However, many published ERAS protocols are convoluted and difficult to apply.
Prior to the introduction of ERAS in plastic surgery, ABR was often seen as a complex procedure, and patients could expect a long postoperative hospital stay with a slow recovery.
In our initial paper, we demonstrated that the application of a simple, inexpensive, early ERAS protocol could reduce patient length of stay (LOS) by more than 1 day for those undergoing unilateral ABR with an abdominal flap. We did this by comparing the historical data from 292 patients [1994–2003] to that of 177 ERAS patients [2006–2011]. Applying an ERAS protocol significantly reduced LOS from 7.4 to 6.2 days (P=0.0002). In 2016, our established ERP setup for ABR with free abdominal flaps (15) was published. Analyzing 16 consecutive patients, we demonstrated a significant reduction in LOS: from 6.2 to 3.1 days (P<0.001). We have just published our 5-year follow-up of 147 unilateral ABRs with abdominal flap, in which a mean LOS of 3 days was achieved. In our department, ERAS is no longer a research tool but the standard of care in microsurgical breast reconstruction.
We here present an overview of ERAS, with recent data selected and based on our personal ERAS experience in ABR with DIEP flaps and LD flaps over the last 10 years. We present the following article in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-26/rc).
Methods
The search for the literature cited in this paper was conducted based on guidelines suggested by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The search was performed by the main author as described in the Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1 March 2021 |
| Databases and other sources searched | PubMed, Embase, Web of Science |
| Search terms used | “ERAS”, “enhanced recovery after surgery”, “breast reconstruction”, “postoperative care”, “breast” |
| Timeframe | 2000–2020 |
| Inclusion and exclusion criteria | All types, English text only |
| Selection process | CB selected all references |
ERAS: implementation and challenges
With millions of operations performed each year worldwide, postoperative complications remain a significant problem in the 21st century. The concept of ERAS, previously known as fast-track surgery, is a peri- and postoperative care concept first described in detail by Kehlet (8) in 1997. ERAS is based on identifying and adjusting important factors that contribute to the successful treatment of a surgical patient. ERAS is a multidisciplinary approach involving the surgeons, anesthesiologists, nurses, and physiotherapists as they manage patient treatment. ERAS standardizes and limits variation in postoperative patient care while providing a multimodal approach to controlling perioperative pathophysiology. It thereby mitigates the risk of organ dysfunction and enhances recovery. The goals of ERAS are to improve postoperative recovery and reduce perioperative risk, LOS, morbidity, and mortality, with the ultimate aim of achieving pain- and risk-free surgery (16). Several studies and meta-analyses comparing the ERAS concept with conventional care have been published in most surgical specialties, including orthopedic surgery, abdominal/hepatic surgery, and gynecology, and all clearly show ERAS to be superior to traditional protocols (17). However, the literature on the use of ERAS in plastic surgery and microsurgical procedures is more limited. Nonetheless, evidence accumulated over the 5 years suggests that ERAS can improve postoperative recovery by shortening LOS and reducing medical complaints without increasing the risk of surgery-related complications and readmissions, even after major reconstructive procedures like ABR (18).
First introduced by Holmstrӧm in 1979, microsurgical breast reconstruction with use of a free abdominal flap has become a well-established practice (19). The procedure has since been modified and today is mostly performed as a perforator-based reconstruction (20), aided by computed tomographic or magnetic resonance angiograms (21-24). The musculocutaneous LD flap was described over a hundred years ago (25) and has been extensively used in ABR since the 1970s (26).
In 2015, we published one of the first reports of an ERP in microsurgery (27); in 2016, we published our final ERP setup for ABR with free abdominal flaps (15), which was followed by the publishing of our experience in applying the same protocol for ABR with LD flaps (18). We showed that by adhering to a few simple, easy-to-measure, functional discharge criteria (FDC), it was possible to safely discharge the patients by the third postoperative day (POD).
An important step in the popularization of ERAS was the establishment of the ERAS Society (28). In 2001, a group of surgeons formed the ERAS study group with the goal of developing perioperative care protocols. The ERAS study group subsequently established a nonprofit international society (the ERAS Society; http://www.erassociety.com/) to further develop the ERAS concept. In 2017, the ERAS Society endorsed a set of guidelines for breast reconstruction (29), which described 18 care elements in the pre-, peri-, and postoperative periods. These included minimal fasting, carbohydrate loading, multimodal pain and nausea prophylaxis, judicious fluid administration, early refeeding, and early ambulation. While useful and relevant, the guidelines highlighted one of the challenges of implementing a clinically effective ERAS: many protocols are overly complicated, often with more than 15 to 25 recommendations required for successful implementation. Extensive guidelines can hinder progress because they require changes that might not be realistic in most hospital departments in terms of either the resources or the staff available. We believe that to ease implementation, the content of an ERP should be limited. One way to achieve this is to define the most impactful elements first.
Because ERAS is a dynamic process, it can originate, evolve, and become successful by including relatively few core elements, as explained below.
Applying the eras principles to ABR
As mentioned above, the numerous interventions recommended in many ERPs makes it hard to apply them in most hospital settings outside large, resource-strong university hospitals. Another challenge is the interpretation of the different studies using ERAS in ABR. In most publications on ERAS in ABR, the patient populations are quite heterogenous, and both primary and secondary, as well as unilateral and bilateral reconstructions, are analyzed interchangeably (30-32). Our considerations are based on studies performed exclusively on unilateral, secondary reconstructions, as these are the most homogenous autologous reconstructions performed and treats the population for whom the ERP principles are the easiest to apply and maintain. Based on our previous studies and the ERAS principles, we suggest that the treatment pathway can be divided into 3 distinct phases: pre-, peri-, and postoperative. Within each phase, we put forward 3 easy-to-apply core elements that we believe will have the greatest impact (Figure 1). These core elements will help achieve results and provide a practical protocol and not simply act as academic exercise. However, it is vital to remember the single most important point when implementing an ERAS: that it is a team effort. For an ERP to succeed, all professional groups involved in the treatment—nurses, physiotherapists, and doctors—must accept and support the changes so the treatment pathway is coherent and uniform. As for the individual core elements, we are aware that not all recommendations can be applied in all centers due to national or regional differences or regulations, but such variation is inevitable.
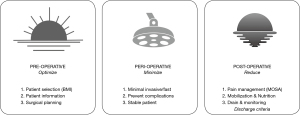
Preoperative core elements (optimize)
A common denominator for the core elements in the preoperative phase is optimization. Patients should be psychologically and physically well prepared. Providing them with sufficient information about the surgery should help enhance the later phases.
Patient selection
Only patients with American Society of Anesthesiologists (ASA) scores of 0 or 1 (33) are accepted for reconstruction. We do not accept patients with more than 1 complication from ASA group 1. Smokers are asked to stop smoking 2 months before the procedure as we do not perform reconstruction in active smokers. It is well-documented that smoking increases the risk of complications, which can include delayed wound healing and infection. In purely elective ABR, except for primary reconstructions, it is acceptable to require the patient to make every possible effort to minimize the risk of complications. The same applies to body mass index (BMI). We do not perform elective surgeries in patients with a BMI >28 kg/m2. Patients with more comorbidities, including obesity (BMI >30 kg/m2), are likely to have more complications and thus be less suited for an ERP (34). The number of complications increases with a higher BMI, and recently, an analysis of over 4,000 DIEP flap reconstructions found more complications in the higher BMI groups and shorter LOS in the lower BMI groups (35).
Patient information
From the initial consultation, the information the patient receives should prepare them for surgery and enhanced recovery. In the early days of microsurgery, patients were often told that this was an advanced procedure that required an extraordinary amount of care and monitoring.
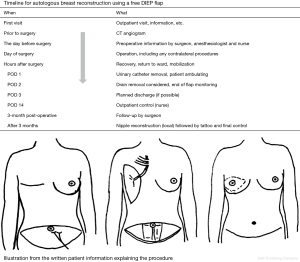
Patients undergoing ABR would receive multiple suction drains and be immobilized for several days. Today, the patient must be involved as an active participant and should receive a realistic overview of the whole treatment pathway. A figure illustrating the treatment timeline and a diagram detailing the operation (Figure 2) will help the patient prepare mentally. They should be carefully informed about the practical aspects of the treatment (e.g., expected arrival time in recovery, timing for the removal of drains, mobilization). We take patients on a mental journey where we explain what is going to happen at every step of the treatment during the hospital admission. For example, patients are told that the first night after surgery will be difficult due to the nurses having to check the flap perfusion every hour, which will make sleep difficult and the patient tired the next morning. Knowing this makes it easier for patients to handle. Due to our knowledge of postoperative pain levels, we can prepare patients for what to expect. They are also told that discharge will happen on the evening of the second POD or the morning of the third. This creates a self-fulfilling prophecy, and, again, helps the patient prepare mentally.
Surgical planning
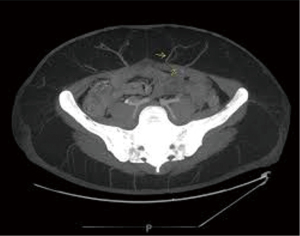
Since 2006, we have routinely performed computed tomography (CT) angiograms in all patients undergoing ABR with abdominal flaps (Figure 3). When selecting the perforator(s) to be used, the main goal is to choose a vessel that allows for both sufficient flap perfusion and the easiest dissection with the shortest possible intramuscular course. During surgery, the strategy is to go directly for the main selected perforator and ignore all other vessels on that side unless another perforator is found to be larger or better placed, despite the initial CT angiogram. This approach allows us to save time raising the flap and therefore shortens the total operating room (OR) time. Based on the CT angiogram, the likelihood of having to convert from a DIEP flap to a muscle-sparing transverse rectus abdominis myocutaneous (MS-TRAM) flap can also be preoperatively determined. The damage done to the rectus muscle from the dissection of 3 or more perforators for a DIEP flap is often comparable to performing an MS-TRAM flap (36). However, any decision to convert the procedure to an MS-TRAM flap should be made as early as possible to save surgical time.
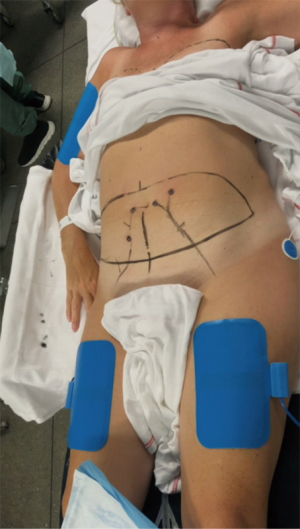
Before starting the procedure, the surgeons assign tasks to each team member (this includes any trainees), so they can work independently. Any special requirements, for example, a preference for short (12 cm) or long (15 cm) micro-instruments, are also decided upon and requested when booking the surgery. Preoperative markings are performed the day before, and the position of the planned perforator is checked with a Doppler ultrasound pencil probe and marked on the skin. Markings for the mastopexy or reduction are also drawn the day before surgery.
Core perioperative elements (minimize)
Minimize is the word that encapsulates the core perioperative elements. Minimizing the surgical stress that the patient is subject to, limiting OR time, and reducing the likelihood of complications all increase the probability of a successful postoperative recovery.
Minimally invasive surgical techniques
There has been a gradual evolution from using muscle flaps to using perforator flaps over the last two decades. By removing as little as possible (preferably none) of the abdominal muscles and conserving the motor nerves to the rectus muscle, damage to the donor site can be minimized (20). Of course, surgeons should be well trained in microsurgery and perform a sufficient annual number of microsurgical procedures to maintain surgical proficiency.
Marginal gain is a concept introduced into microsurgery by professor Venkat Ramakrishnan although it was originally coined by Sir David John Brailsford, a British cycling coach. The concept revolves around having everything under control and functioning at optimum levels while striving toward continuous betterment by focusing on small improvements in any conceivable area 1% at a time. Professor Ramakrishnan et al. “process mapped” the entire surgical process of performing ABR with a DIEP flap. By breaking down the operation into 100 streamlined steps, they enhanced operative efficiency without compromising outcomes (37).
We have successfully implemented other aspects, such as being highly verbal throughout the operation and informing anesthesiologists about the progress and upcoming steps of the procedure. Muscular relaxation during the flap elevation should be interrupted as soon as the fascia is closed. We close the umbilical hole in the flap before moving it to the recipient area and place the suction drain in the recipient area before performing the anastomosis. By considering each small step, each can be improved and the surgery performed more quickly and safely.
Prevention of surgery-related complications
We always use a 2-team approach: one team (usually a consultant and a trainee) will raise the flap, while the other team will prepare the recipient vessels and perform corrections on the contralateral side (mastopexy/reduction). With this setup, symmetrizing surgery can be carried out in parallel operating processes without affecting overall operative times.
The anastomosis is performed by the surgeon who prepares the recipient vessels. We routinely employ 3 diathermy devices, each equipped with monopolar and bipolar diathermy. This requires preoperative planning for the placement of electrodes and an understanding of how the diathermies are directed to each surgical field (Figure 4). This is most often not recommended by the manufacturers but may depend on the brand of diathermy equipment. However, after operating with this setup for over a decade, we have yet to experience any related technical problems. Meticulous hemostasis allows us to use a single abdominal drain, and we refrain from the use of any fibrin glue or quilting sutures. Perioperative antibiotics and measures to prevent thromboembolic (TE) complications should be used.
Stable patient (thermo-, fluid, and pain regulation)
During surgery, when several areas are being worked on at the same time, the patient is very exposed and at risk of hypothermia. They should be placed on a heating blanket, as this will help to keep them normothermic. Fluid replacement should be conservative, and blood products should not be needed. Close teamwork with the anesthesiologist responsible for the microsurgical unit is crucial.
Core postoperative elements (reduce)
The primary focus in the postoperative phase should be on reducing the amount of time spent in bed and in hospital and limiting the time that the patient has a urinary catheter and drains inserted. These goals are supported by the following 3 core elements.
Effective dynamic pain treatment
Multimodal opioid-sparing analgesia (MOSA) is one of the central aspects of ERAS in ABR. A synergetic combination of analgesics and mechanisms that affect different sites in the nervous system results in a lower rate of adverse effects than do higher doses of an individual analgesic. Our published MOSA (38) includes a standard oral cocktail of a COX-2 inhibitor (celecoxib, 200 mg/12 hourly; STADA Nordic, Herlev, Denmark), gabapentin (300 mg/8 hourly), and paracetamol (1 g/6 hourly). Opioids are only administered on request. Aspirin (150 mg) is prescribed 1 day before surgery and for the first 14 PODs. Patients receive standard thromboprophylaxis (3,500 IU of low-molecular-weight heparin (Innohep, Celgene Corp., Boulder, CO, USA) from the day of surgery until discharge. Antibiotics are given only during surgery. The decision to use a COX-2 inhibitor instead of nonsteroidal anti-inflammatory drugs (NSAIDs) is founded on both our clinical results and the well-documented effects of NSAIDs on thrombocyte aggregation. Due to the blockade of prostaglandin synthesis at the COX-1 receptor, NSAIDs can increase the risk of bleeding from the operative site and the gastrointestinal mucosa. Prior to our study, documentary evidence regarding the use of COX-2 inhibitors after free flap surgery was extremely limited due to the concerns about TE complications, which followed the withdrawal of rofecoxib (Vioxx) from the market (39-41). At the time, the only relevant published study suggested a flap loss rate of 29% when the patients were treated with COX-2 inhibitors (42). We demonstrated that a MOSA with a COX-2 inhibitor does not increase flap loss when given postoperatively for no longer than a week and that COX-2 inhibitors may be superior to NSAIDs as they carry a smaller risk of postoperative hematomas.
Early ambulation and oral nutrition
Patients are encouraged to ambulate as early as possible. Preoperative fasting will leave the patient energy-depleted after surgery. Therefore, oral nutritional intake starts on the evening following the procedure. The urinary catheter is removed on the morning of the first POD, and a supportive bra and abdominal compression are worn constantly during the first 3 weeks.
Due to the preoperative information they receive, patients know that the first day is going to be challenging, but by the second POD, all patients are eating and free of pain [visual analogue scale (VAS) <4] (15). Furthermore, both the MOSA and early oral nutrition can help reduce the incidence of ileus (43). Although immobilization is a major pathogenic factor for deep vein thrombosis and pulmonary embolism, we have not observed these complications in any of our ABR patients after the implementation of our ERAS protocol. This is most likely due to early postoperative mobilization, which is supported by reports on ERAS in hip and knee arthroplasty that suggest long-term TE prophylaxis may not be required (44-46).
Rational use of drains and flap monitoring
In analyzing our ERAS data, we found that the main reason for LOS >3 days after ABR was the use of drains (unpublished data, article under review). Individual preferences of the doctor doing the rounds will often determine when drains are removed, with output ranging from <10 to <100 mL. According to our ERAS protocol, nurses remove the drains without consulting the doctors on day 2 if total production is less than 50 mL, and on day 3 if total production is less than 100 mL (15). This strategy eliminates any personal preferences regarding drain removal and is supported by the literature: Miranda et al. (47,48) found no difference in total complications, seroma, dehiscence, or hematoma rates between late and early drain removal for ABR with both LD and DIEP flaps.
Flap monitoring is performed every hour in the first 24 hours, and every 2 hours for the following 24 hours. While most vascular complications will occur within the first 24 hours after microsurgery, the benefits of early detection gained from reliable flap monitoring over 48 hours may well outweigh the additional cost and relatively low workload associated with the extra 24 hours of monitoring (49).
FDC
A final concept that supports these core elements is the development of FDC. A well-defined set of functional endpoints will make it clear to all staff exactly how long the patient is to remain hospitalized. FDC can vary depending on the surgical specialty and specific procedure. For example, our FDC for ABR are different from our FDC for microsurgical head and neck reconstruction (50). In the case of ABR, we use a simple set of 7 functional parameters, defined to help establish when the patient is ready for discharge (15). The FDC can be evaluated once or twice a day, and when all 7 criteria are met, the patient should be discharged unless there is another specific reason for extending their stay. In such a case, the cause should be registered. The parameters of the FDC are the following: mobilization (more than 4 hours/day); oral feeding (eating normally), drains (all drains removed), freedom from pain (VAS score less than or equal to 4), flap monitoring (laser doppler/hand held ultrasound doppler monitoring discontinued at 6 pm on POD 2), personal hygiene (ability to shower and use the toilet), and gastrointestinal function (patient has gastrointestinal function).
Although the most commonly reported aspect of ERAS is LOS, there has been recent skepticism about its relevance as an appropriate marker of having achieved a pain- and risk-free operation (51). While easily measurable, it is only valuable if precise discharge criteria, similar to our 7 points, and the destination of discharge are taken into account.
The 9 core elements described above have been used primarily for DIEP flap reconstructions although similar results have also been obtained when using the LD flap. Although the surgical procedures are different, the principles remain the same, and most of the core elements are identical, the exception being that no CT angiograms are performed in LD flap reconstructions.
Our experience and the future
When preparing to implement an ERAS protocol, it is important for any department to review their traditional care regimen and procedural results to establish a baseline and ascertain what challenges they typically face during postoperative hospitalization. In 2006 we reviewed our traditional recovery after surgery (TRAS) experience for ABR (7). After a preparatory pilot study, the full ERAS protocol was implemented on January 1, 2006.
The first 2 publications on enhanced recovery in plastic surgery, both focusing on microsurgical breast reconstruction, were published by Batdorf et al. and by our group within a few months of each other in 2015 (27,52). Both studies reported a statistically significant reduction of LOS by about 1 day in the ERAS group compared to the TRAS group.
Prior to these 2 studies, ABR was considered a complex and advanced procedure. Patients would have multiple suction drains and an epidural catheter, be hospitalized for extended periods of time, mobilized late, and prepared for a late discharge (Table 2).
Table 2
| Pre-ERAS (<2006) | Post-ERAS (>2006) | |
|---|---|---|
| Drains (No.) | 4 | 2 |
| Drains removed | 30 mL or POD 7 | <50 mL or POD 3 |
| Flap monitoring period | 3 days* | 2 days** |
| Epidural | Yes, removed POD 3 | No |
| Urinary catheter removed | Day 3 | Day 1 |
| Mobilization | Day 3 | Day 0/1 |
| Planned discharge | Day 7 | Day 3 |
*, every 30 min, 72 hours; **, every hour in the first 24 hours and every second hour for the following 24 hours. ERAS, enhanced recovery after surgery; POD, postoperative day.
Our first 5-year analysis [2006–2011] consisted of 177 unselected consecutive patients treated with unilateral ABR, with use of an MS-TRAM or DIEP flap. This ERAS group was then compared to the 277 patients treated under the TRAS. Results were modest but clear: by introducing a simple peri- and postoperative care program, it was possible to reduce LOS after microsurgery by at least 1 day (from 7 to 6 days) with no increase in complications or flap loss (27). Over the following years, we developed the ERAS further and were the first to define a set of FDC for ABR with free abdominal flaps (15). Our final ERP setup was published in 2016, and we demonstrated that LOS after ABR with DIEP flaps could be reduced to approximately 3 days. Since our follow-up study was published in 2016, reports of using ERAS in ABR have steadily grown in number and acceptance.
Two of the challenges of interpreting studies using ERP in ABR are the heterogeneity of many patient populations and the need to clearly distinguish between primary and secondary as well as between unilateral and bilateral reconstructions. Another issue is assessing the stability of the ERAS protocol results when they are no longer used in a closely monitored research setup but rather as the standard of care.
We recently reviewed our 5-year results of using our ERAS as the standard of care and found them to be consistent with our early experience. More than 80% of the patients undergoing unilateral secondary breast reconstruction with a free abdominal flap were able to be discharged directly to their home on the third POD. Discharging patients with drains on the second POD could further reduce LOS since drains are the main reason for a prolonged LOS.
In our unit, the main alternative to a DIEP for ABR is the pedicled LD flap. We also use the thoracodorsal artery perforator (TAP) flap, but since the majority of our reconstructions are secondary, the TAP perforator can be damaged, thus necessitating the use of the full LD.
Using the same ERAS protocol and MOSA (38), we expanded our implementation to cover breast reconstructions with LD flaps and a permanent implant (18). We reviewed our past results (53) and compared these data to those of the ERAS program for LD reconstructions as well those from another surgical team who continued to perform LD reconstructions without implementing the ERAS (TRAS). LOS was significantly shorter in the ERAS group (3.2 days) when compared to the historical (6.9) and TRAS (6.3) groups. Drains were removed significantly faster in the ERAS group (day 3.9) in comparison to the historical (day 6.3) and TRAS (day 7.0) groups.
In summary, our standard ERAS protocol reduced LOS from 6 to 3 days without increasing complications in unilateral breast reconstructions using both DIEP and LD flaps.
We are currently using our ERAS protocol for primary and bilateral ABR and awaiting the results. Patients in these cases face additional surgical procedures—mastectomy and/or two free flap reconstructions—thus generating greater surgical stress and, in theory, a higher risk of complications and an extended LOS.
Finally, we have recently described the most common postoperative challenges for recovery in patients who have undergone microvascular reconstruction for head and neck cancer using a modified version of our ERP for this complex procedure (50). These findings now serve as the core of our ERP for microsurgical reconstructions.
As seen above, in uncomplicated cases, LOS after ABR should be around 3 days. It might be possible to reduce this to just 2 days in large international centers, but we are unlikely to be able to reduce it much further due to the nature and extent of the surgery.
National and regional differences and traditions that are not based on science can hinder the implementation of even the best protocols, and profit is sometimes dependent on longer hospital stays, which works against an early discharge.
However, with health care under constant pressure to deliver improved results despite financial restrictions, significant potential exists for improving the clinical pathway for a wide variety of surgical procedures, including ABR. There is a need for more evidence-based procedure-specific studies to evaluate the effects of individual interventions on relevant procedures. ERAS recommendations should be well-documented from rigorous, relevant studies, and these studies should focus on the core elements of enhanced recovery to benefit the patients. Protocols and studies should specify the type of procedure (unilateral or bilateral), the destination of the patient at discharge, and the indication for surgery (primary or secondary). This would allow readers to easily differentiate patients and compare the results.
The goals of ERAS are to reduce the incidence of complications and readmissions, and to improve patient quality of life after surgery. Future investigations should begin to shift the focus from reducing LOS to the avoidance of post-discharge problems.
The concept of ERAS is becoming more widely accepted and applied in various areas of reconstructive surgery. We are currently using or investigating the possibilities of applying ERAS in our primary and bilateral ABR, in our microsurgical head and neck reconstructions, and in our orthoplastic collaborations. Further analysis in other aspects of plastic surgery with long, complex pathways, such as pressure sores and perhaps even transgender surgeries, will define the future role of ERAS.
Conclusions
The concept of ERAS can and should be applied to ABR with both free (DIEP) and pedicled (LD) flaps. The goal is to see improved patient recovery with no increase in flap loss or complications and a reduced LOS with discharge on POD 3 after ABR.
To achieve this, any implementation of an ERAS protocol should focus on team effort, the 9 procedure-specific core elements, and the FDC. Our ERAS protocol is no longer a research tool but the standard of care in ABR.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction—The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-26/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-26/coif). The series “Breast Reconstruction—The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Korvenoja ML, Smitten K, Asko-Seljavaara S. Problems in wearing external prosthesis after mastectomy and patient's desire for breast reconstruction. Ann Chir Gynaecol 1998;87:30-4. [PubMed]
-
Society DC 2020 . Available online: https://www-dep.iarc.fr/NORDCAN/DK/StatsFact.asp?cancer=200&country=208 - Hoejvig JH, Pedersen NJ, Gramkow CS, et al. Delayed two-stage breast reconstruction: The impact of radiotherapy. J Plast Reconstr Aesthet Surg 2019;72:1763-8. [Crossref] [PubMed]
- Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: one-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg 2000;106:1014-25; discussion 1026-7. [Crossref] [PubMed]
- Damen TH, Morritt AN, Zhong T, et al. Improving outcomes in microsurgical breast reconstruction: lessons learnt from 406 consecutive DIEP/TRAM flaps performed by a single surgeon. J Plast Reconstr Aesthet Surg 2013;66:1032-8. [Crossref] [PubMed]
- Patel NG, Ramakrishnan V. Microsurgical Tissue Transfer in Breast Reconstruction. Clin Plast Surg 2017;44:345-59. [Crossref] [PubMed]
- Bonde CT, Christensen DE, Elberg JJ. Ten years' experience of free flaps for breast reconstruction in a Danish microsurgical centre: an audit. Scand J Plast Reconstr Surg Hand Surg 2006;40:8-12. [Crossref] [PubMed]
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [Crossref] [PubMed]
- Adamina M, Kehlet H, Tomlinson GA, et al. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery 2011;149:830-40. [Crossref] [PubMed]
- Mertz BG, Kroman N, Williams H, et al. Fast-track surgery for breast cancer is possible. Dan Med J 2013;60:A4615. [PubMed]
- Schultz NA, Larsen PN, Klarskov B, et al. Evaluation of a fast-track programme for patients undergoing liver resection. Br J Surg 2013;100:138-43. [Crossref] [PubMed]
- Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003;362:1921-8. [Crossref] [PubMed]
- Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189-98. [Crossref] [PubMed]
- Kehlet H. Fast-track colorectal surgery. Lancet 2008;371:791-3. [Crossref] [PubMed]
- Bonde CT, Khorasani H, Elberg J, et al. Perioperative Optimization of Autologous Breast Reconstruction. Plast Reconstr Surg 2016;137:411-4. [Crossref] [PubMed]
- Kehlet H. Enhanced postoperative recovery: good from afar, but far from good? Anaesthesia 2020;75:e54-61. [Crossref] [PubMed]
- Smith TW Jr, Wang X, Singer MA, et al. Enhanced recovery after surgery: A clinical review of implementation across multiple surgical subspecialties. Am J Surg 2020;219:530-4. [Crossref] [PubMed]
- Højvig JH, Kehlet H, Bonde CT. Enhanced recovery after breast reconstruction with a pedicled Latissimus Dorsi flap-A prospective clinical study. J Plast Reconstr Aesthet Surg 2021;74:1725-30. [Crossref] [PubMed]
- Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg 1979;13:423-27. [Crossref] [PubMed]
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Masia J, Clavero JA, Larrañaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [Crossref] [PubMed]
- Masia J, Clavero JA, Larrañaga J, et al. Preoperative planning of the abdominal perforator flap with multidetector row computed tomography: 3 years of experience. Plast Reconstr Surg 2008;122:80e-1e. [Crossref] [PubMed]
- Masia J, Larrañaga J, Clavero JA, et al. The value of the multidetector row computed tomography for the preoperative planning of deep inferior epigastric artery perforator flap: our experience in 162 cases. Ann Plast Surg 2008;60:29-36. [Crossref] [PubMed]
- Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [Crossref] [PubMed]
- Tansini I. Sopra il mio nuovo processo sli amputazione della mammella. Gaz Med Ital 1906.
- McCraw JB, Penix JO, Baker JW. Repair of major defects of the chest wall and spine with the latissimus dorsi myocutaneous flap. Plast Reconstr Surg 1978;62:197-206. [Crossref] [PubMed]
- Bonde C, Khorasani H, Eriksen K, et al. Introducing the fast track surgery principles can reduce length of stay after autologous breast reconstruction using free flaps: A case control study. J Plast Surg Hand Surg 2015;49:367-71. [Crossref] [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Temple-Oberle C, Shea-Budgell MA, Tan M, et al. Consensus Review of Optimal Perioperative Care in Breast Reconstruction: Enhanced Recovery after Surgery (ERAS) Society Recommendations. Plast Reconstr Surg 2017;139:1056e-71e. [Crossref] [PubMed]
- Afonso A, Oskar S, Tan KS, et al. Is Enhanced Recovery the New Standard of Care in Microsurgical Breast Reconstruction? Plast Reconstr Surg 2017;139:1053-61. [Crossref] [PubMed]
- Sindali K, Harries V, Borges A, et al. Improved patient outcomes using the enhanced recovery pathway in breast microsurgical reconstruction: a UK experience. JPRAS Open 2018;19:24-34. [Crossref] [PubMed]
- Rendon JL, Hodson T, Skoracki RJ, et al. Enhanced Recovery after Surgery Protocols Decrease Outpatient Opioid Use in Patients Undergoing Abdominally Based Microsurgical Breast Reconstruction. Plast Reconstr Surg 2020;145:645-51. [Crossref] [PubMed]
- Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology 1978;49:233-6. [Crossref] [PubMed]
- Fischer JP, Sieber B, Nelson JA, et al. Comprehensive outcome and cost analysis of free tissue transfer for breast reconstruction: an experience with 1303 flaps. Plast Reconstr Surg 2013;131:195-203. [Crossref] [PubMed]
- Heidekrueger PI, Fritschen U, Moellhoff N, et al. Impact of body mass index on free DIEP flap breast reconstruction: A multicenter cohort study. J Plast Reconstr Aesthet Surg 2021;74:1718-24. [Crossref] [PubMed]
- Bonde CT, Lund H, Fridberg M, et al. Abdominal strength after breast reconstruction using a free abdominal flap. J Plast Reconstr Aesthet Surg 2007;60:519-23. [Crossref] [PubMed]
- Sharma HR, Rozen WM, Mathur B, et al. 100 Steps of a DIEP Flap-A Prospective Comparative Cohort Series Demonstrating the Successful Implementation of Process Mapping in Microsurgery. Plast Reconstr Surg Glob Open 2019;7:e2016. [Crossref] [PubMed]
- Bonde C, Khorasani H, Hoejvig J, et al. Cyclooxygenase-2 inhibitors and free flap complications after autologous breast reconstruction: A retrospective cohort study. J Plast Reconstr Aesthet Surg 2017;70:1543-6. [Crossref] [PubMed]
- White C. Further action taken on COX 2 inhibitors in Europe. BMJ 2005;331:12. [Crossref] [PubMed]
- White PF. Changing role of COX-2 inhibitors in the perioperative period: is parecoxib really the answer? Anesth Analg 2005;100:1306-8. [Crossref] [PubMed]
- Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 2005;352:1081-91. [Crossref] [PubMed]
- Al-Sukhun J, Koivusalo A, Törnwall J, et al. COX-2 inhibitors and early failure of free vascular flaps. N Engl J Med 2006;355:528-9. [Crossref] [PubMed]
- Kehlet H. Postoperative ileus--an update on preventive techniques. Nat Clin Pract Gastroenterol Hepatol 2008;5:552-8. [Crossref] [PubMed]
- Petersen PB, Kehlet H, Jørgensen CC, et al. Safety of In-Hospital Only Thromboprophylaxis after Fast-Track Total Hip and Knee Arthroplasty: A Prospective Follow-Up Study in 17,582 Procedures. Thromb Haemost 2018;118:2152-61. [Crossref] [PubMed]
- Samama CM. Fast-Track Procedures in Major Orthopaedic Surgery: Is Venous Thromboembolism Prophylaxis Still Mandatory? Thromb Haemost 2019;119:3-5. [Crossref] [PubMed]
- Samama CM. Postoperative Venous Thromboembolism Prophylaxis: Changes in the Daily Clinical Practice, Modified Guidelines. Semin Thromb Hemost 2020;46:83-8. [Crossref] [PubMed]
- Miranda BH, Amin K, Chana JS. The drain game: abdominal drains for deep inferior epigastric perforator breast reconstruction. J Plast Reconstr Aesthet Surg 2014;67:946-50. [Crossref] [PubMed]
- Miranda BH, Amin K, Chana JS. The drain game: back drains for latissimus dorsi breast reconstruction. J Plast Reconstr Aesthet Surg 2014;67:226-30. [Crossref] [PubMed]
- Shen AY, Lonie S, Lim K, et al. Free Flap Monitoring, Salvage, and Failure Timing: A Systematic Review. J Reconstr Microsurg 2021;37:300-8. [Crossref] [PubMed]
- Højvig JH, Pedersen NJ, Charabi BW, et al. Microvascular reconstruction in head and neck cancer - basis for the development of an enhanced recovery protocol. JPRAS Open 2020;26:91-100. [Crossref] [PubMed]
- Joshi GP, Kehlet H. Enhanced Recovery Pathways: Looking Into the Future. Anesth Analg 2019;128:5-7. [Crossref] [PubMed]
- Batdorf NJ, Lemaine V, Lovely JK, et al. Enhanced recovery after surgery in microvascular breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:395-402. [Crossref] [PubMed]
- Højvig JB, Bonde CT. Breast reconstruction using a latissimus dorsi flap after mastectomy. Dan Med J 2015;62:A5155. [PubMed]
Cite this article as: Bonde CT, Højvig J. The development of autologous breast reconstruction and the impact of enhanced recovery after surgery (ERAS): a narrative review. Ann Breast Surg 2023;7:18.