The impact of radiotherapy in pre-pectoral implant-based breast reconstruction: a narrative review
Introduction
Background
Pre-pectoral implant-based reconstruction (PIBR) is becoming increasingly popular due to the preservation of normal chest wall anatomy. It avoids the surgical morbidity associated with chest wall muscle dissection; eliminating animation deformity and replacing the new breast implant in its normal anatomical plane where the breast tissue was removed (1).
The main indication for this technique is immediate breast reconstruction following mastectomy for cancer or for risk-reducing surgery. PIBR can potentially be very useful in breast revision surgery, particularly to correct animation deformity and capsular contracture.
This procedure can be considered in any patient who would normally be suitable for an implant breast reconstruction. It may be preferable in athletes, may be more suitable for non-smokers, those with no history of previous radiotherapy (RT) and patients with grade 1 or 2 ptosis. Breasts with grade 3 ptosis and anticipated breast weight more than 500 g can potentially be offered this technique with a dermal sling.
The procedure is generally offered to patients who are fit and well, with no major comorbidities or well-controlled minor comorbidities, body mass index (BMI) <35 kg/m2, no previous RT damage to the chest wall and with a resectable tumour. Patients with a type 3 breast according to the Breast Tissue Coverage Classification developed by Rancati et al. (that is a thickness of the sub-cutaneous layers at pre-operative digital mammogram more than 2 cm) are particularly suited to PIBR (2).
The technique is generally avoided in tumours involving the skin, chest wall muscle, locally advanced ones, inflammatory breast cancers and in those with an increased chance of chest wall recurrence (3).
It is usually used with acellular dermal matrix (ADM) to cover it under well vascularised skin flaps. Complete or partial coverage ensures that the ADM/mesh is placed exactly in the required position with minimal fixation. A few studies have suggested a decreased incidence of capsular contracture following complete coverage though randomized controlled studies are lacking (4-7).
Anterior coverage with ADM results solely in coverage of implant anteriorly, so the posterior aspect of the prosthesis is formed by the underlying pectoralis major muscle. The anterior coverage requires greater attention to technique in positioning the implant and ADM compared to complete coverage with ADM. However, it may more closely mimic the function of the pectoral muscle in implant reconstruction in reducing implant visibility along the upper pole. Many surgeons believe there may be an increased risk of implant rotation and herniation though specific published evidence is not available (3).
In large ptotic breasts where a skin reduction pattern is employed, a dermal flap along with the ADM/mesh can constitute a complete pre-pectoral pocket. The presence of a dermal flap contributes to lower pole soft tissue coverage while the ADM/mesh completes the coverage superiorly if required (8). This may be a suitable option in ptotic breasts in patients who have a high BMI, though with an increased risk of perioperative complications (9).
Different biological and synthetic meshes are available in the market and their use may be based on local availability, patient and surgeon preference and the cost-effectiveness. It is well known that biological meshes undergo collagen remodelling and re-vascularisation, while synthetic meshes integrate through fibrosis (3). Good quality long-term comparative studies of different meshes and ADMs remain to be published. Nevertheless, a wide variety of meshes and ADMs are in common usage globally.
Furthermore, the data available so far are limited regarding the effect of post-mastectomy radiotherapy (PMRT) including capsular contracture, implant loss and cosmesis. Similarly, comparative data concerning the effect of previous RT are scarce in the literature. This review looks to collate the information we have regarding RT in PIBR thus far. We present the following article in accordance with the Narrative Review reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-132/rc).
Objective
This review aimed to assess the effect of RT on the outcome of PIBR.
Materials, methods and results
The main outcome of this narrative review included both major and minor complications of PIBR in patients who received RT either pre- or post- mastectomy.
To identify all available studies, a systematic search was performed in all electronic databases (PubMed, Web of Science, Scopus, EMBASE). We used medical subject headings (MeSH) and free-text words using the following search terms in all possible combinations: “prepectoral implant reconstruction”, “prepectoral breast reconstruction”, “breast implant reconstruction”, and “radiotherapy”. The last search was performed in October 2021 (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 15 October 2021 |
| Databases and other sources searched | PubMed, Web of Science, Scopus, EMBASE |
| Search terms used | “Prepectoral implant reconstruction”, “prepectoral breast reconstruction”, “breast implant reconstruction”, and “radiotherapy” |
| Timeframe | Studies published between 2011 and October 2021 |
| Inclusion and exclusion criteria | Inclusion criteria: all studies, over the last 10 years, that reported or mentioned effect of radiotherapy on patients who had PIBR were included. The search strategy was limited to articles written in English language |
| Exclusion criteria: papers regarding animal studies, editorials and case series with less than 10 patients were excluded | |
| Selection process | Two independent authors analyzed each article and performed the data extraction independently. Duplicate studies were removed. Further they reviewed independently the eligibility of studies in abstract form and in full text by assessing if the inclusion criteria and outcome measures were met. Discrepancies were resolved by consensus |
Study selection
Two investigators independently reviewed articles for eligibility based on the study titles and abstracts, and studies that met the inclusion criteria were retrieved for full-text assessment, data extraction, and inclusion in the review. All disagreements were resolved by consensus.
Data collection process
Two independent authors analyzed each article and performed the data extraction independently. Duplicate studies were removed. Further they reviewed independently the eligibility of studies in abstract form and in full text by assessing if the inclusion criteria and outcome measures were met. Discrepancies were resolved by consensus.
Eligibility criteria
We included all studies, over the last 10 years, that reported or mentioned effect of RT on patients who had PIBR.
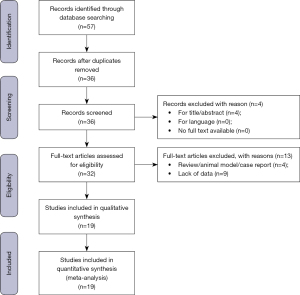
The search strategy was limited to articles written in English language; moreover, papers regarding animal studies, review articles, editorials, and case series with less than 10 cases were excluded (Figure 1).
The quality of each included study was assessed with the Newcastle-Ottawa Scale (NOS): it contains eight items, categorized into three domains: (I) selection of study (four points); (II) comparability of groups (two points); (III) ascertainment of exposure and outcomes (three points) for case-control and cohort studies, respectively. A star system is used to allow a semi-quantitative assessment and researchers assign up to a maximum of nine points (Table 2). Nineteen studies met inclusion criteria and their related results to our review were summarised in Table 3.
Table 2
| References | Selection | Comparability | Outcome assessment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 1 | 2 | 3 | |||
| Mathew J et al., 2021 | * | * | * | * | ** | * | * | |||
| Thuman JM et al., 2021 | * | * | * | * | * | * | ||||
| Coyette M et al., 2021 | * | * | * | * | ** | * | * | * | ||
| Sinnott CJ et al., 2021 | * | * | * | * | ** | * | * | |||
| Razzouk K et al., 2020 | * | * | * | * | * | * | * | |||
| Masià J et al., 2020 | * | * | * | * | * | * | ||||
| Safran T et al., 2020 | * | * | * | * | ** | * | * | * | ||
| Polotto S, 2020 | * | * | * | * | ** | * | ||||
| Chandarana M et al., 2020 | * | * | * | * | ** | * | * | * | ||
| Bilezikian JA et al., 2020 | * | * | * | * | ** | * | * | * | ||
| Sobti N et al., 2020 | * | * | * | * | ** | * | * | * | ||
| Momeni A et al., 2019 | * | * | * | * | ** | * | * | * | ||
| Reitsamer R et al., 2019 | * | * | * | * | ** | * | * | * | ||
| Viezel-Mathieu A et al., 2020 | * | * | * | * | ** | * | * | * | ||
| Sigalove S, 2019 | * | * | * | * | ** | * | * | * | ||
| Casella D et al., 2019 | * | * | * | * | ** | * | * | * | ||
| Sbitany H et al., 2019 | * | * | * | * | ** | * | * | * | ||
| Sinnott CJ et al., 2018 | * | * | * | ** | * | * | ||||
| Elswick SM et al., 2018 | * | * | * | * | * | * | * | |||
*, 1 star point; **, 2 stars points. NOS, Newcastle-Ottawa Scale.
Table 3
| First author, year | Patients/breasts, n | Radiation, n | Hematoma, n | Seroma, n | Infection, n | Capsular contractures, n | Wound dehiscence, n | Implant loss, n |
|---|---|---|---|---|---|---|---|---|
| Mathew J et al., 2021 | 85 breasts | 21 | 1 | 1 | 1 | – | 8 | 2 |
| Thuman JM et al., 2021 | 109 breasts | 44 | 0 | 20 (12*) | 15 (10*) | 12 (7*) | 2 (1*) | 5 (2*) |
| Coyette M et al., 2021 | 50 | 12 (3 pre-Mx) | 2 | 1 | 1 | – | 2 | 2 |
| Sinnott CJ et al., 2021 | 369/592 | 71 (26 pre-Mx) | 1 | 5 (4*) | 30 (12*) | 33 (17*) | 5 (2*) | 30 (13*) |
| Razzouk K et al., 2020 | 136 | 136 | – | 7 | 1 | – | – | 3 |
| Masià J et al., 2020 | 1,186/1,450 | 198 (45 pre-Mx) | 31 (4*) | 111 (22*) | 70 (10*) | 31 (10*) | 67 (8*) | 94 (22*) |
| Safran T et al., 2020 | 201/313 | 96 (38 pre-Mx) | 9 | 7 | 7 | – | 2 | 8 |
| Polotto S, 2020 | 186 | 28 | 1 | 16 (2*) | 9 | 4 (3*) | 5 | 3 (1*) |
| Chandarana M et al., 2020 | 324/406 | 77 (15 pre-Mx) | 10 | 29 | 13 | 1 | 8 | 26 |
| Bilezikian JA et al., 2020 | 131 | 22 (10 pre-Mx) | – | – | 10 (1*) | 131 | – | 10 (1*) |
| Sobti N et al., 2020 | 20 | 20 | 0 | 0 | 0 | 12 | – | 0 |
| Momeni A et al., 2019 | 40/69 | 12 (4 pre-Mx) | – | 4 | 3 | – | – | 1 |
| Reitsamer R et al., 2019 | 134/200 | 58 (26 pre-Mx) | 8 (3*) | 29 | 1* | – | – | 7 (2*) |
| Viezel-Mathieu A et al., 2020 | 39/60 | 12 | 2 | 0 | 2 (1*) | – | – | 0 |
| Sigalove S, 2019 | 33/52 | 34 | – | 1* | – | 0 | 1* | 1* |
| Casella D et al., 2019 | 397/521 | 131 (71 pre-Mx) | 1 | 5 | 12 | 19 | 7 | 16 |
| Sbitany H et al., 2019 | 175 breasts | 40 (14 pre-Mx) | 2 | 2 | 11 | – | 4 | 4 |
| Sinnott CJ et al., 2018 | 274/426 | 45 | 0* | 1 | 12 (3)* | 22 (9*) | 4 (1*) | 17 (3*) |
| Elswick SM et al., 2018 | 54/93 | 54 | 2 (1*) | 5 (3*) | 13 (10*) | 1* | 4 (1*) | 1* |
Complications are referred to all patients enrolled; number of complications referred to irradiated patients out of total number of complications are reported in parentheses with *; complications referred to irradiated patients are reported with *. Mx, mastectomy.
Discussion and summary
Relatively few studies were undertaken and their outcomes were released in the last few years. Some of them were designed to address impact of RT. Others just mentioned its effect on their cohort.
Overall, early experiences revealed that adjuvant RT is well tolerated with PIBR (10). However, patients may need further interventions, including lipomodelling following PMRT. Sbitany et al. observed that PIBR had similar outcomes in the setting of PMRT to that of sub-muscular ones (11). Furthermore, the authors found no difference in complication rates between pre-pectoral and sub-pectoral implant-based breast reconstruction. Despite the results being based on single surgeon experience; their outcome was taken significantly in writing of the joint consensus guideline from European and USA breast and plastic and reconstructive surgeons which was published in May 2019 (3) when authors agreed that PMRT appears to be well tolerated in immediate PIBR with no excess adverse effects. Hence, they recommended that PIBR could be offered to patients who need planned PMRT. Especially that this procedure is used with ADM that can lead to decrease rate of capsular contracture. Furthermore, ADM provides protective effect. Two studies were referred to in regards ADM role by authors to reach such consensus (6,12).
Furthermore, the consensus that was reached revealed that the experience with previous RT is limited and influenced mainly by the degree of damage caused by RT and patient preference (3). Hence, it is recommended that each patient should be individually assessed.
Since the time of editing the consensus, a few publications were released concerning PIBR and previous RT. In 2020, Razzouk et al. found that lipofilling combined with PIBR after RT is a method that yields a satisfactory cosmetic outcome with a low complication rate and which can be an attractive alternative to flap-based reconstruction in some irradiated patients. The study was multicentric but retrospective (13).
Recently, Sinnott et al. published results of their study. It was a retrospective one (14). They aimed to compare the impact of pre-mastectomy vs. post-mastectomy RT on outcomes after pre-pectoral breast reconstruction. Patients were recruited over a 9-year period. They found that in pre-pectoral implant breast reconstruction, pre- and post-mastectomy RT were associated with higher rates of infection and implant loss compared with non-radiated patients. PMRT was associated with a higher rate of capsular contracture compared with non-radiated patients, and a comparable rate of capsular contracture compared with pre-mastectomy radiation therapy patients. Pre-mastectomy RT was associated with a higher rate of seroma compared with post-mastectomy RT and non-radiated patients.
Coyette et al. similarly concluded that prior breast irradiation does not increase postoperative complication rates. However, they had small cohort; 64 mastectomies and only 3 of them had pre-mastectomy RT (15).
In early 2020, Chandarana et al. published the results of National Braxon Audit Study Group (BAG) from UK. They found no relation between irradiation and rate of complications. However, they recommended longer follow-up duration to study the impact of RT (16). Their results were based on 406 mastectomies. Sixty-two of them had post-mastectomy RT. Moreover, only 15 had pre-mastectomy RT. It is worth to be mentioned in such a non-randomised study there may be significant selection bias in choice of operative technique.
These results were included in the international Braxon Audit Group (iBAG) study which represents the largest evidence on PIBR with mesh up to now according to our review. It was published in August 2020 (17). Their results did not reveal statistically significant correlation between RT and postoperative complications, except for capsular contracture, that even so did not reach a high percentage (about 5%). This result was based on studying in details the effect of irradiation on 198 reconstructed breasts (45 received pre-mastectomy irradiation and 159 had PMRT). Despite that it is a retrospective study. This audit is valuable as it was international and multicentric. They collected data of experience on PIBR with Braxon ADM from 30 centres across Spain, UK, and Italy.
iBAG results agreed with Elswick et al. results (18) that was published in 2018 regarding impact of PMRT on PIBR and that capsular contracture is higher in irradiated group. However, this study was limited by a short follow-up after definitive implant placement (9 months).
In the same year 2018, Sinnott et al. compared the impact of PMRT in pre-pectoral vs. sub-pectoral procedures (19). They found that sub-pectoral reconstruction showed higher capsular contracture rate than PIBR especially in irradiated breasts. Worth to be mentioned that the two groups were not matched. PIBR procedures number outweighed that of sub-pectoral ones (426 vs. 163). Also, the number of irradiated reconstructed breast in PIBR was 56 when it was on 23 in the sub-pectoral group. Yet PIBR outcome was more favourable regarding effect of PMRT. Again, this is a non-randomized retrospective study.
Furthermore, Sobti et al. studied capsular contracture rate in direct to implant sub-pectoral vs. pre-pectoral reconstruction (20). They found capsular contracture rate greater in the first group. In fact, they reported that sub-pectoral implant placement was nearly 4 times as likely to result in capsular contracture (P<0.01). They had small cohort of 47 patients (81 breasts). Thirty-two of them had PIBR. Of which 20 were irradiated. The remaining 49 breasts that had sub- pectoral reconstruction, 27 of them were irradiated.
On the other hand, Momeni et al. (21), who published their results just 1 year earlier than Sobti et al., had a slightly larger cohort of 80 patients. Forty of them had PIBR. Most of them had an expander to implant procedure (two-stage procedure). In both sub- and pre-pectoral group, a small number had RT; 12 in pre-pectoral group and 14 in the sub-pectoral one. Their results revealed a favourable outcome in a well-matched patient population in immediate pre-pectoral tissue expander insertion with anterior ADM coverage compares to sub-pectoral tissue expander placement. The matched-pair analysis made their results somewhat more valuable though still falls short of a well powered randomised trial.
Casella et al. compared two-stage reconstruction with direct to implant (DTI) PIBR (22). They found that PMRT is significantly associated to a higher risk of developing surgical complications in DTI PIBR. That was contradicted by Reitsamer et al., who reported no significant complications in irradiated group that were reconstructed with PIBR using one-stage direct to implant with ADM/mesh (23). They commented that capsular contractures grade III or IV could not be observed in patients with previous RT or in patients with RT to the reconstructed breast.
Viezel-Mathieu et al. also compared pre-pectoral approach with a sub-pectoral one (24). However, they used direct to implant procedure (one-stage) in the pre-pectoral group (39 patients) vs. two-stage implant reconstruction in the sub-pectoral group (38 patients) making comparisons more difficult. Again, this study detailed the PMRT in each group and reported no poor outcomes in those who had PIBR, i.e., 12 patients out of 39. In favour of this study that both groups were comparable in size and demographics including age, diabetic and smoking status, and receiving neoadjuvant/adjuvant therapy. However, only 12 patients in PIBR received PMRT when 20 where irradiated in the sub-pectoral group. However, as it is not our aim to compare sub- with pre-pectoral procedure. This unlikely will affect our outcome.
Sigalove’s study warned against PIBR in previously irradiated breasts (25). The author concluded that for patients who have been radiated in the past, care should be exercised when considering PIBR without a concurrent vascularized muscle flap. However, immediate PIBR followed by RT appears to be well tolerated, with no excess risk of adverse outcomes, at least in the shorter-term follow-up reported. The study had 52 PIBR procedures. Thirty-four of these reconstructed breasts had PMRT. They had only one reconstruction loss and another one had seroma. Their average follow-up was 25 months. The author acknowledged that longer follow-up is required. Furthermore, that their outcome is preliminary.
Thuman et al. published their outcomes in June 2021 (26). They compared impact of RT on pre-pectoral vs. sub-muscular implant-based reconstruction. The retrospective study was run over 7 years and included 387 breasts. Almost two third of them had a sub-muscular approach (287 breasts). They found that the pre-pectoral group (109 breasts) had a significantly lower incidence of reconstructive failure than sub-muscular placement regardless of RT status. They concluded that PIBR performs clinically better than sub-muscular in non-radiated patients compared with radiated ones; however, no statistical significance was identified. The authors recommended a larger study to get more statistically significant difference if any, which seems appropriate. The two groups were not matched. Only 44 reconstructions were irradiated in pre-pectoral group compared to 141 ones in the sub-muscular group. This mismatch weakens this comparison. However, if PIBR group to be considered solely in our review. It shows that this procedure has tolerance for PMRT.
Mathew published a retrospective study designed to compare short to medium term outcome of pre-pectoral vs. sub-pectoral implant-based reconstruction with ADM (27). He included 109 breasts reconstruction, 85 PIBR and 24 sub-pectoral. Whilst the effect of RT was not a primary end point of the study, yet the cohort is included in our review. Thirty-six patients had RT. Twenty-one of them belonged to pre-pectoral group. Two of 36 patients who received RT lost their implants compared with one in 73 who did not receive RT. Furthermore, eight patients in RT group had fat grafting compared with 17 in those who did not receive RT, though these events did not achieve statistical significance. Hence, he commented that RT did not have a significant adverse outcome in the short to medium follow-up in both groups, but its long-term impact has not been addressed. Mismatched two groups and relatively short follow-up period for average 2 years make it difficult to draw firm conclusions from the study.
Safran et al. experience showed no significant differences in complication rates for patients who had PIBR and PMRT (28). Their cohort was of 201 patients and 313 breasts. Only 58 of them received PMRT. This result agreed with Polotto et al. outcome that had even a smaller cohort of irradiated patients, i.e., 28 out of 186. But they reported slightly higher rate of capsular contracture in irradiated group compared to non-irradiated ones (29).
Last but not least, Bilezikian et al. reported that PIBR represents a beneficial shift in breast reconstruction regardless of irradiation and other risk factors. They linked their conclusion to intraoperative evaluation of the vascular integrity of mastectomy skin flaps with fluorescent imaging. They used tissue expanders whenever skin flaps had poor vascularity.
A cohort of 131 patients whom they followed only for 21 months were included. Eight of them had tissue expanders. Twelve had PMRT and ten had pre-mastectomy RT. Only one of the irradiated group had implant loss. When non-irradiated group had nine. Furthermore, all the patients regardless of radiotherapy had capsular contracture grade one or two (30).
Our review is limited being a narrative one. There is a paucity of studies about PIBR in general. We found only 7 studies which, met criteria that were designed to study impact of RT in PIBR (11,13,14,18,19,26,29).
Furthermore, less is available about impact of RT on PIBR with stratification one-stage vs. two-stage and use of ADM or not. Only one study did not use ADM (15) and a direct comparison of reconstruction with and without ADM in the same reconstructive plane, whilst perhaps difficult to design and recruit to, would give enormously valuable information. Moreover, most of studies were retrospective, single-centre and had short-term follow-up durations. Hence, longer follow-up is needed to better understand the impact of RT in PIBR.
In summary, previous RT is not a contraindication to the procedure though evidence on the relative complication risk for these patients is contradictory. Furthermore, PMRT appears to be well tolerated in immediate PIBR with no excess adverse effects especially when used with ADM/mesh at least in the short-term. More studies are needed to examine the long-term impact of RT with this technique and indeed the long-term outcomes for pre-pectoral implants in terms of cosmesis, practicalities of revision over time and the need for salvage autologous reconstruction at a later stage.
There is a clear paucity of suitably powered randomized studies in these patients. The large number of meshes/ADMs available and lack of standardised surgical techniques make comparisons difficult and conclusions must be drawn on low level evidence. Randomised trials are badly needed.
Acknowledgments
We would like to thank Dr. Jamil Najjar for language editing assistance, Dr. Nicola Rocco for inspiring the work, and Dr. Manas Kumar Dube for data collection.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nicola Rocco, Giacomo Montagna and Giuseppe Catanuto) for the series “New Perspectives in Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-132/rc
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-132/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-132/coif). The series “New Perspectives in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg 2012;68:346-56. [Crossref] [PubMed]
- Rancati AO, Angrigiani CH, Hammond DC, et al. Direct to Implant Reconstruction in Nipple Sparing Mastectomy: Patient Selection by Preoperative Digital Mammogram. Plast Reconstr Surg Glob Open 2017;5:e1369. [Crossref] [PubMed]
- Vidya R, Berna G, Sbitany H, et al. Prepectoral implant-based breast reconstruction: a joint consensus guide from UK, European and USA breast and plastic reconstructive surgeons. Ecancermedicalscience 2019;13:927. [Crossref] [PubMed]
- Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon® acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg 2017;87:493-8. [Crossref] [PubMed]
- Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 2015;68:162-7. [Crossref] [PubMed]
- Cheng A, Lakhiani C, Saint-Cyr M. Treatment of capsular contracture using complete implant coverage by acellular dermal matrix: a novel technique. Plast Reconstr Surg 2013;132:519-29. [Crossref] [PubMed]
- Schmitz M, Bertram M, Kneser U, et al. Experimental total wrapping of breast implants with acellular dermal matrix: a preventive tool against capsular contracture in breast surgery? J Plast Reconstr Aesthet Surg 2013;66:1382-9. [Crossref] [PubMed]
- Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-Reduction Breast Reconstructions with Prepectoral Implant. Plast Reconstr Surg 2016;137:1702-5. [Crossref] [PubMed]
- Thuman J, Freitas AM, Schaeffer C, et al. Prepectoral Wise-Pattern Staged Implant-Based Breast Reconstruction for Obese or Ptotic Patients. Ann Plast Surg 2019;82:S404-9. [Crossref] [PubMed]
- Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg 2011;128:1162-9. [Crossref] [PubMed]
- Sbitany H, Gomez-Sanchez C, Piper M, et al. Prepectoral Breast Reconstruction in the Setting of Postmastectomy Radiation Therapy: An Assessment of Clinical Outcomes and Benefits. Plast Reconstr Surg 2019;143:10-20. [Crossref] [PubMed]
- Seth AK, Hirsch EM, Fine NA, et al. Utility of acellular dermis-assisted breast reconstruction in the setting of radiation: a comparative analysis. Plast Reconstr Surg 2012;130:750-8. [Crossref] [PubMed]
- Razzouk K, Fitoussi A, Al Khori N, et al. Breast Reconstruction Combining Lipofilling and Prepectoral Prosthesis after Radiotherapy. Plast Reconstr Surg Glob Open 2020;8:e2659. [Crossref] [PubMed]
- Sinnott CJ, Pronovost MT, Persing SM, et al. The Impact of Premastectomy Versus Postmastectomy Radiation Therapy on Outcomes in Prepectoral Implant-Based Breast Reconstruction. Ann Plast Surg 2021;87:S21-7. [Crossref] [PubMed]
- Coyette M, Coulie J, Lentini A, et al. Prepectoral immediate breast reconstruction with polyurethane foam-coated implants: Feasibility and early results in risk-reducing and therapeutic mastectomies. J Plast Reconstr Aesthet Surg 2021;74:2876-84. [Crossref] [PubMed]
- Chandarana M, Harries SNational Braxon Audit Study Group. Multicentre study of prepectoral breast reconstruction using acellular dermal matrix. BJS Open 2020;4:71-7. [Crossref] [PubMed]
- Masià J. iBAG Working Group. The largest multicentre data collection on prepectoral breast reconstruction: The iBAG study. J Surg Oncol 2020;122:848-60. [PubMed]
- Elswick SM, Harless CA, Bishop SN, et al. Prepectoral Implant-Based Breast Reconstruction with Postmastectomy Radiation Therapy. Plast Reconstr Surg 2018;142:1-12. [Crossref] [PubMed]
- Sinnott CJ, Persing SM, Pronovost M, et al. Impact of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann Surg Oncol 2018;25:2899-908. [Crossref] [PubMed]
- Sobti N, Weitzman RE, Nealon KP, et al. Evaluation of capsular contracture following immediate prepectoral versus subpectoral direct-to-implant breast reconstruction. Sci Rep 2020;10:1137. [Crossref] [PubMed]
- Momeni A, Remington AC, Wan DC, et al. A Matched-Pair Analysis of Prepectoral with Subpectoral Breast Reconstruction: Is There a Difference in Postoperative Complication Rate? Plast Reconstr Surg 2019;144:801-7. [Crossref] [PubMed]
- Casella D, Di Taranto G, Onesti MG, et al. A retrospective comparative analysis of risk factors and outcomes in direct-to-implant and two-stages prepectoral breast reconstruction: BMI and radiotherapy as new selection criteria of patients. Eur J Surg Oncol 2019;45:1357-63. [Crossref] [PubMed]
- Reitsamer R, Peintinger F, Klaassen-Federspiel F, et al. Prepectoral direct-to-implant breast reconstruction with complete ADM or synthetic mesh coverage - 36-Months follow-up in 200 reconstructed breasts. Breast 2019;48:32-7. [Crossref] [PubMed]
- Viezel-Mathieu A, Alnaif N, Aljerian A, et al. Acellular Dermal Matrix-sparing Direct-to-implant Prepectoral Breast Reconstruction: A Comparative Study Including Cost Analysis. Ann Plast Surg 2020;84:139-43. [Crossref] [PubMed]
- Sigalove S. Prepectoral breast reconstruction and radiotherapy-a closer look. Gland Surg 2019;8:67-74. [Crossref] [PubMed]
- Thuman JM, Worbowtiz N, Jain A, et al. Impact of Radiation on Implant-Based Breast Reconstruction in Prepectoral Versus Submuscular Planes. Ann Plast Surg 2021;86:S560-6. [Crossref] [PubMed]
- Mathew J. Short- to Medium-term Outcome of Prepectoral versus Subpectoral Direct-to-implant Reconstruction using Acellular Dermal Matrix. Plast Reconstr Surg Glob Open 2021;9:e3747. [Crossref] [PubMed]
- Safran T, Al-Halabi B, Viezel-Mathieu A, et al. Direct-to-Implant, Prepectoral Breast Reconstruction: A Single-Surgeon Experience with 201 Consecutive Patients. Plast Reconstr Surg 2020;145:686e-96e. [Crossref] [PubMed]
- Polotto S, Bergamini ML, Pedrazzi G, et al. One-step prepectoral breast reconstruction with porcine dermal matrix-covered implant: a protective technique improving the outcome in post-mastectomy radiation therapy setting. Gland Surg 2020;9:219-28. [Crossref] [PubMed]
- Bilezikian JA, Tenzel PL, Bebb GG, et al. The Broad Application of Prepectoral Direct-to-Implant Breast Reconstruction with Acellular Dermal Matrix Drape and Fluorescent Imaging in a Community Setting. Plast Reconstr Surg 2020;145:291-300. [Crossref] [PubMed]
Cite this article as: Taher W, Nadeem M, Velotti N, Nava MB, Hamilton S. The impact of radiotherapy in pre-pectoral implant-based breast reconstruction: a narrative review. Ann Breast Surg 2023;7:26.