Breast-conserving surgery and immediate autologous fat transfer with or without neoadjuvant treatment: indications, technique, cosmetic and oncologic outcomes
Introduction
Breast cancer conservative treatment was validated many years ago following the publications of Umberto Veronesi and Bernard Fisher (1-3). Although one of the objectives of breast conserving surgery is to reduce mastectomy rates, it must also avoid cosmetic sequelae.
Compliance with the basic principles of breast conservation, which consist of: sound knowledge of the anatomy, assessment of the volume to be resected including the margins relative to the glandular volume, attempt to predict potential sequelae of adjuvant radiotherapy (4) and training in cosmetic and reconstructive surgery techniques such as mastoplasty for medium or large sized breasts, does not assure satisfactory results in every case and may result in sequelae that are difficult to correct in approximately 30% of patients (5).
Oncoplastic techniques designed to prevent such sequelae were described over 30 years ago and with every passing day, new procedures are published for this purpose with varying degrees of aggressiveness (6). The experience of several groups and their publications have led to a systematic approach to the indications based on breast size, tumor location and tumor volume/breast volume ratio (7) for these procedures to be more reproducible by the majority of breast surgeons.
Fat transfer for breast augmentation was reported for the first time in 1895 by Czerny (8) who transplanted a dorsal lipoma to restore sequelae of a mastectomy. Bircoll (9), used liposuction for the first time in 1987 to perform breast lipofilling with the same purpose.
In 1987, the Ad Hoc Committee of the American Society of Plastic Surgeons (ASPS) reviewing new procedures banned the use of fat grafting concerned about the potential sequelae and calcifications that might interfere with subsequent cancer detection (10).
This concern lost strength when new publications proved that, with advanced diagnostic equipment, the experience of breast imaging specialists and the comparison with sequelae that other procedures such as reduction mastoplasties produced, breast lipotransfer not only did not generate significant diagnostic difficulties, rather the doubtful BIRADS III and IV images were less frequent with these techniques (11,12).
Later on, with the arrival of a systematic approach published by Coleman (13), breast fat transfer returned to the scene and it was Rigotti et al. (14) in 2007 who used it to repair severe sequelae associated to radiotherapy such as actinic ulcers as well as sequelae from breast cancer conservative surgery.
In 2009, the Fat Graft Task Force (ASPS) broadened the indications of this breast reconstruction procedure, but maintained the restrictions for breast augmentation (15).
Subsequently Petit et al. questioned the oncologic safety of fat grafting only for subgroups of patients with a history of intraepithelial neoplasms treated with conservative surgery, where the correction of sequelae with lipofilling might generate more risk of local events in women <50 years, with a high-grade neoplasm and Ki-67 ≥14 (16). These concerns were solved with a longer term follow-up of the same group, where the recurrence curves showed to be similar to those of the control group without lipotransfer (17).
Consequently, thereon, lipofilling was considered as a safe procedure in terms of diagnostic follow up and its non-influence on the generation of new oncologic events. It became popular as first choice technique to correct sequelae of conservative treatment both for minor defects and for some major defects that in the past had to be repaired with more aggressive techniques with the advantage of yielding good outcomes with fewer complications and without the need to resort to implants or flaps (18).
In 2016, Moltó García et al. published a small experience attempting to associate immediate fat-transfer to breast tumor resection with an unrefined technique and a one-year follow-up, with good results (19).
In 2015, Biazus et al. from the Hospital de Clínicas in Porto Alegre, Brazil (HCPA) published the first experience of immediate lipotransfer in conservative treatment with a standardized technique in 20 cases with good results and a low complication rate with a follow up of 13 to 19 months (20).
Subsequently, the same group investigated oncologic safety comparing a group of 27 patients vs. a control group of 167 patients without immediate lipotransfer, finding no differences in oncologic events with a follow up of 36 months (21). Their results were confirmed by Biazus in a third prospective, non-randomized and non-controlled publication of 65 patients where he came to the same conclusion in a follow up of 40.8 months (22).
Based on everything we have learnt and our extensive experience in the use of lipotransfer in breast reconstruction, we started using immediate fat grafting after breast conserving surgery in 2017.
The objective of this study is to analyze the usefulness of immediate lipotransfer both in primary breast-conserving surgery; and in a second subgroup of patients with indication for conservative surgery after neoadjuvant treatment.
The aim in both subgroups was to assess patient selection criteria, details of surgical technique, cosmetic outcomes, complications and the local and distant oncological outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-131/rc).
Methods
Between June 2017 and January 2021, 91 patients were included from the Mastology Department of the Instituto de Oncología “Ángel H Roffo” of the University of Buenos Aires undergoing breast conservative surgery and breast reconstruction with autologous fat transfer within the same surgery (BCT-IMM-LF) and performed by the same surgical team.
This prospective, non-randomized and non-controlled study included patients with invasive stage I, II and III tumors with indication for conservative treatment. Of the 91 patients included, 61 underwent primary surgery without prior treatment (BCT-AFT) and 30 had surgery after neoadjuvant treatment as dictated by the clinical, imaging and immunohistochemical assessment of the tumor (BCT-AFT-NEO). The exclusion criteria were in situ tumors, tumors with indication for conservative surgery but with skin involvement caused by the disease and inflammatory tumors that respond to neoadjuvant treatment.
The study was conducted in accordance with the Helsinki Declaration (as revised in 2013). The study was approved by the research and ethics committee of the Mastology Unit of the Roffo Institute (Approval No. 02/16 UM-IAR) and the patients were included to participate in the study and gave their written consent by signing the informed consent form before their surgery, after having received the explanations of the procedure and understanding the possible consequences associated with the injection of autologous fat grafting in the breast. They were also informed that further investigation may be necessary if questionable images appear with studies such as digital mammography, ultrasonography or magnetic resonance imaging (MRI) of the breast and possible percutaneous biopsies in the resection and graft areas.
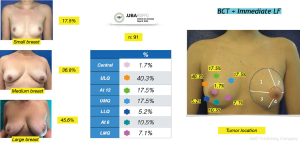
In the primary group (BCT-AFT) all the patients were stage I (15%) and II (85%) and their immunohistochemical profile was Luminal A in 40 patients (65.5%) and Luminal B in 21 patients (34.5%). In the group with indication for neoadjuvant treatment (BCT-AFT-NEO) 23 patients were stage III (76.5%) and the rest were stage II (24.5%), and their immuno-histochemical profile was Luminal A in 2 patients (6.5%), Luminal B in 17 patients (56.5%), triple negative in 8 patients (26.5%) and Her2+ in 3 patients (10%).
In terms of patient selection, we took into account the breast volume-tumor volume ratio and if conservative treatment had a high potential of poor cosmetic results or if a mastectomy would be required, to allow testing, the efficacy or not of immediate lipofilling in these patients.
The following factors were analyzed: age, weight, body mass index (BMI), tumor location and size, tumor size/breast volume ratio, histopathological and immunohistochemical characteristics of the tumor and assessment of the fatty tissue donor areas, as well as tumor volume and weight and fat graft volume. Postoperative breast and donor site complications, local and distant disease recurrences, overall survival and disease-free survival were analyzed. Patient follow-up was performed with clinical examination, digital mammography, breast ultrasound and in selected cases an MRI with gadolinium, as recommended by The Breast Imaging Reporting and Data System (BIRADS), 5th edition (23).
The surgical technique involved complete resection of the tumor approached by cosmetic incisions according to tumor location (circumareolar or radial), measurement, weighing and assessment of the resected volume, placement of the surgical specimen on a sterile plate, orientation and marking of the margins and inking of the entire surface with Indian ink to guide the intraoperative frozen section examination by the pathologist (Figure 1). A thorough examination of the tumor and margins was done to check if intraoperative margin widening was required. All the surgeries continued after confirming that the tumor did not contact the dye. The sentinel lymph node was explored with a single marking procedure (dye or radioisotope) in primary patients and with the dual method (dye plus radioisotope) in post neoadjuvant treatment patients.

In the post NEO patients, axillary exploration of 4 sentinel lymph nodes is taken as the minimal number of nodes to decide on the best course of action. Axillary dissection was performed in the cases with metastatic lymph nodes detected in the frozen biopsy.
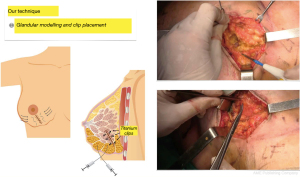
As soon as the pathology examination confirms that the margins are negative, in line with the SSO/ASTRO guidelines (consensus guidelines), we proceed first, to remodel the residual mammary gland by dissecting two centimeters in the retro glandular and pre glandular spaces of both resection margins; 4 to 6 titanium clips are placed on the resection margins to facilitate radiotherapy (Boost) and the gland is sutured edge to edge with resorbable material, leaving behind significant deformities in terms of shape and projection of the breast as well as skin retractions produced by the pull of Cooper’s ligaments (Figure 2).
The most frequently used technique to transfer fat was published by Coleman (24). As a result of our own lipofilling experience we made some modifications to this technique. Before we proceed with liposuction, we inject Klein’s solution in the donor area (50 mL of 1% lidocaine solution) (500 mg), 1 mL of 1:1,000 epinephrine solution (1 mg), 1,000 mL of 0.9% NaCl solution, and 12.5 mL of 8.4% NaH2 CO3 solution (12.5 mEq). The choice of different donor areas is determined depending on the patient’s anatomy and the likelihood of having to perform additional procedure in the future to correct residual defects.
If there are several alternative donor sites, we prefer to aspirate the abdomen first and the flanks as a second choice. The subcutaneous fat is harvested manually with 60 cc syringes and vacuum connected to a 3 mm cannula with 2 orifices in the same axis. Liposuction is performed with gentle care to preserve the superficial layer of adipose tissue avoiding secondary depressions and the sample is always taken symmetrically so as not to alter the patient’s cosmetic appearance.
The material obtained is left to settle in the 60 cc syringes for 15 minutes and subsequently, the supernatant oil and the previously injected Klein’s solution are disposed, until we are left with fatty tissue only. It is then transferred via an intermediary tube to 5 and 10 cc syringes, depending on the defect to be corrected, which are connected to a grafting cannula. We use the Roger Khouri blunt, curved cannula (14 G =1.63 mm), with a spatulate-shaped round tip, with a single anterior hole in the concavity of the needle close to the tip where the fat outpours. The cannula concavity allows easy access from a distance to the entire breast anatomy including the pectoralis major muscle, thus minimizing the risk of puncture injuries in the thoracic wall.
The injection and modeling technique to correct a resection defect is performed in 4 steps customizing the amount of fat injected and the application sites to each case, according to the sequelae, the shape of the breast and symmetry.
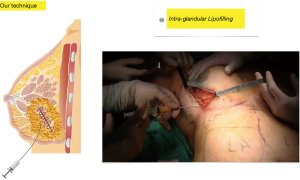
First step: fat is injected into the entire mammary gland adjacent to the resection area in several directions and in several planes in a retrograde fashion following linear paths without producing cavities, the caliper of the cannula (1.6 mm) and the single orifice avoids excessive buildup and spread of the graft and it additionally reduces the likelihood of intravascular injection (Figure 3).
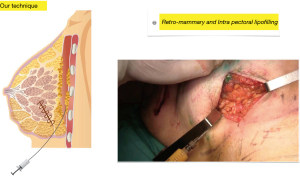
Second step: using the same technique and the same cannula, fat is injected into the pectoralis major muscle to increase breast projection whenever the resection has altered it, during this step, fat is also injected into the retro mammary space with the same aim (Figure 4).
Third step: fat is injected in the pre-glandular subcutaneous space adjacent to the skin incision, a length of 3 to 4 cm surrounding the entire incision, and in several planes and directions in a retrograde fashion. In this step, if necessary, additional intra-glandular injections can be made (Figure 5).
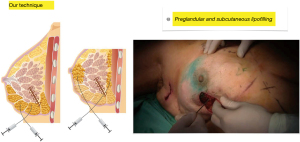
Fourth step: with the patient in the semi-sitting position with her arms close to her body, we inspect the irregularities produced by Cooper’s ligaments that pull the skin between the lipofilled areas and proceed to perform multiple percutaneous subcisions using an 18 G needle blade (Rigotomies) until a harmonious result is achieved, free of retractions that deform the breast contour. In this step it may be necessary to add a few subcutaneous or even subdermal fat injections (nano-lipofilling) with more diluted fat injected with fine hypodermic needles to improve skin trophism in selected cases to optimize the result (Figure 6).
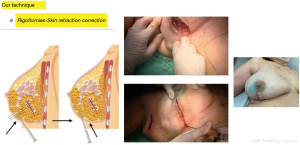
Before proceeding to suture the subcutaneous cellular tissue and skin, we examine the patient in the sitting position with her arms close to her body to check for shape and volume defects and correct them, if necessary with additional lipofilling. At this time, we assess if it will be necessary to add more volume to the operated breast taking into account the spontaneous partial fat resorption or radiotherapy-induced retraction and volume loss. An overcorrection of approximately 30% to 40% in excess of the resected tissue volume is transferred without altering the breast shape and contour.
After each lipofilling stage, a gentle manual massage is performed to spread the fat evenly to avoid the development of oily lakes and cysts.
Finally, we proceed to subcutaneous and intradermal skin suturing with slow absorbable material.
None of the patients had to undergo simultaneous symmetry correction procedures (breast augmentation or reduction) in the opposite breast.
After 6 and 18 months of having completed the radiation treatment, patients and physicians from the Mastology Department who did not participate in the surgery filled out an aesthetic outcome questionnaire (20) evaluating the shape, volume and symmetry of the breasts, with a score from 0 to 10 for each item. The average of both opinions was tabulated as fair or poor when the score was 0–5, good: 6–8 and very good: 9–10.
The results were photographically recorded with the patients consent before surgery, in the immediate postoperative period and at different stages of their follow up with special interest at the 6- and 18-month time points for cosmetic evaluation and analysis of likely changes between the two time points. This iconography was stored in the Department of Mastology database with exclusive access to the professionals involved.
All the patients with or without neoadjuvant therapy received radiotherapy as per the institutional guidelines based on international guideline recommendations. patients with indications for radiotherapy only at the breast volume level received accelerated hypo fractionated WBI with 3DCRT or IMRT techniques at 40.05 Gy/2.67 Gy/Fx for 15 fractions with a subsequent boost at the lumpectomy site at doses of 10 Gy/2 Gy/Fx.
Patients with indication for radiotherapy at the lymph node level received standard WBI with IMRT or 3DCRT techniques at 50 Gy/2 Gy/2 Gy/Fx for 25 fractions, followed by a subsequent boost at the lumpectomy site at doses of 10 Gy/2.5 Gy/Fx; and at the axilla and supraclavicular level the dose prescribed was 50 Gy/2 Gy/Fx.
The primary patients (BCT-AFT) received specific adjuvant treatments according to their immunohistochemical status. Patients with indication for neoadjuvant treatment (BCT-AFT-NEO) received specific treatment according to their immunohistochemical status prior to surgery. Their response was evaluated to indicate breast conservation with mammography, ultrasound and MRI with gadolinium and subsequently, adjuvant treatment was indicated according to the Residual Cancer Burden (RCB) grade and the post-surgical immunohistochemical examination.
Oncological follow-up was performed with a clinical examination every 3 months for the first 2 years and every 6 months after that period. Digital mammography and ultrasound were performed every 6 months during the first 2 years, starting with the first follow up 6 months after having completed their radiotherapy. After 2 years they were followed with annual mammography and ultrasound. Before every scan, the imaging specialist received a card with diagrams showing the fat injection sites.
Locoregional and distant oncologic events were assessed in both groups with neoadjuvant therapy (BCT-AFT-NEO) and without neoadjuvant therapy (BCT-AFT).
Statistical analysis
A descriptive and exploratory analysis of the surveyed variables was carried out. Summary measures were used according to the nature of the variable and its distribution, using count and percentage for categorical variables, mean for variables with normal distribution, and median and interquartile range for continuous variables that do not present normal distribution. For the analysis of time to an event, analyzing the time to progression, Kaplan-Meier graphs and log-rank tests were applied. These data are not reported because there were no oncological events during follow-up. The aesthetic result was evaluated by means of a questionnaire of the cosmetic result reported by observation of the patient and of a professional who did not participate in the procedure and tabulated from 0 to 10.
Results
At the time of surgery, the mean age of the patients in the (BCT-AFT) group was 53.7 years (range, 38–69 years) and 47.8 years (range, 33–62 years) in the (BCT-AFT-NEO) group. The pathology examination reported pT1 and pT2 in all the patients included in the primary surgery group with a mean tumor size of 23.5 mm. Assessing tumor size in the post neoadjuvant therapy BCT-AFT-NEO patient group, was challenging due to their varied response to treatment according to their immuno-histochemical profile and were categorized and reported following the RCB criteria. All the patients included in this group had a clinical o imaging tumor mass in the preoperative assessment, but their tumor size/breast volume ratio allowed indicating breast conservative surgery and required an oncoplastic procedure to avoid sequelae. The postoperative assessment showed residual breast tumor in the 2 Luminal A tumors, in 90% of the Luminal B tumors, 1 Her2+ patient and 4 patients with triple negative tumors.
Most of the tumors in the whole series were invasive ductal carcinomas (70.9%), 17.5% were invasive lobular carcinomas and 2.7% were invasive medullary carcinomas. There was a predominance of histologic grade 2, absence of lymphovascular invasion and absence of intracanalicular extension. The reoperation rate to correct margins reported as involved in the deferred pathology examination was 4.3%.
The patients in the BCT-AFT group had a negative axilla in 59% of the cases, 1 to 3 nodes involved in 15%, more than 4 axillary nodes in 15% and micro metastases in 11% of the cases. In the BCT-AFT-NEO group, all of the patients with a negative axilla before the treatment persisted with a negative axilla post-NEO at the sentinel node and were not subjected to axillary lymphadenectomy. When the axilla was positive pre-NEO, 65% of the patients, had one or several positive sentinel nodes post-treatment and in those cases axillary lymphadenectomy was performed.
The BMI was 25 to 30 in 90% of the patients and 30 to 35 in 6% of them (Table 1).
Table 1
| Characteristics | Immediate lipofilling—no NEO | Immediate lipofilling—NEO |
|---|---|---|
| N | 61 | 30 |
| Mean age | 53.7 years (range, 38–69 years) | 47.8 years (range, 33–62 years) |
| Tumor diameter 1/2 | 23.5 mm | NA |
| Tumor type | 70.9% invasive ductal carcinomas, 17.5% invasive lobular carcinoma, 2.7% invasive medullary carcinoma | |
| SLN-negative axilla | 59% | 100% in pre-NEO negative axillae, 35% in post-NEO axillae |
| SLN-positive axilla | 41% | 65% in post-NEO axillae |
| Reoperation rate | Reoperations to clear margins reported as involved by the deferred pathology report: 4.3% | |
| BMI | 90% had a BMI between 25 and 30 and 6% had a BMI between 30 and 35 | |
NEO, neoadjuvant chemotherapy; NA, not applicable; SLN, sentinel lymph node; BMI, body mass index.
The distribution in terms of quadrants treated and breast sizes is shown in Figure 7. There was a predominance of the upper lateral quadrant (40.3%) followed by the upper medial quadrant (17.5%). and at the 12 o’clock position (17.5%). The large breasts were the most frequently corrected with this method (45.6%). The mean volume of fat injected was 85 g (range, 45–225 g) and the volume to be injected was determined taking into account the intraoperative assessment of the cosmetic outcome as explained in the surgical technique description. All of the patients included in this study were managed with a single lipofilling procedure at the same time the tumor was resected. The volume of fat injected in every case exceeded at least double of the resection specimen volume, avoiding overcorrecting the resection defect to prevent complications and poor outcomes. Special care was taken to preserve the breast shape, to improve skin irregularities and maintain or correct symmetry.
Whenever a reoperation was required to widen margins, the previous breast incision was used, the corrected margins were examined intraoperatively by frozen biopsy and a secondary correction with lipofilling was made adjacent to the resection area in 2 cases.
The procedures were in most cases performed as outpatients with an average hospital stay of 8 hours, and exceptionally of 24 hours. The overall rate of surgical complications of the complete series was 5.49%, there were 2 hematomas, 1 cellulitis that subsided with antibiotic treatment, 1 sequela with a depression in the donor area of the thigh and 1 necrosis of the abdominal donor area that required excision and a dermolipectomy.
Sixty percent of the patients were followed up with images at 6 and 18 months after surgery. There were few diagnostic doubts categorized as BIRADS III and IV in this group of patients, two cases with clustered micro calcifications in mammography and 2 nodules found by ultrasound that were watched and did not evolve. Only two patients presented with lesions categorized as BIRADS IV on ultrasound and required needle biopsies that were reported as an oily cyst and cytosteatonecrosis.
The mean follow-up of the patients was 25.21 months (6–47 months) in the (BCT-AFT group) and 20.9 months (6–48 months) in the BCT-AFT-NEO group. None of the patients with or without NEO presented locoregional recurrences during the study period. Two patients presented distant metastases, one without NEO (Luminal B: bone metastases) and one with NEO (triple negative: lung metastases) 2.19%. Both patients had axillary lymph node metastases in the deferred pathology.
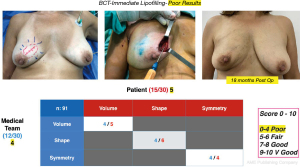
Cosmetic outcome evaluations were performed at 6 and 18 months after-surgery, the results according to the average score of the patient’s self-assessment and of an independent observer were good and very good in 89% of the cases (Figure 8) and fair or poor in 11% (Figure 9).
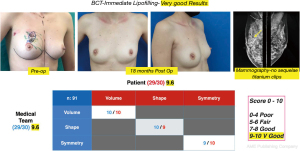
It is interesting to note that when the cosmetic evaluation results exclusively at the 18-month time point were analyzed, good and very good results were found in 4 cases in the score of patients who presented the operated breast with good cosmetic structure in terms of shape, projection, contour, regularity and skin quality, but with different degrees of asymmetry secondary to actinic sequelae in the treated breast (Figure 10).
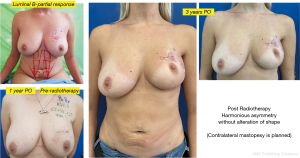
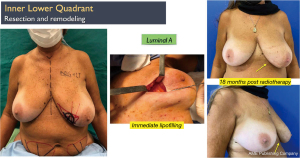
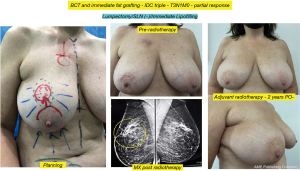
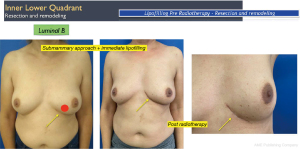
Another interesting observation is the evaluation of the cosmetic results in unfavorable situations such as large breasts, upper or medial quadrants, which in general, are indications for therapeutic mastoplasty. Satisfactory results beyond 85% were obtained in this subgroup (Figures 11,12), and in small breasts in the lower quadrants, particularly on the medial side (Figure 13).
Discussion
Conservative treatment of breast cancer since its inception has had three fundamental objectives: reducing indication of mastectomies, oncological safety and preservation of cosmetic outcome.
In the early experiences using different conservative techniques, that included resecting part of the breast skin when wide quadrantectomies were performed resulted in severe defects in terms of shape, symmetry and asymmetry of the nipple-areola complex, which worsened with radiotherapy (25).
As a consequence of these sequelae, techniques for the correction of these defects were described and further on, there were publications of preventive techniques that required knowledge of plastic and reconstructive surgery and involved not only the repair of the affected breast but the simultaneous correction of symmetry as well. In 2002, Petit et al. (26) published two years of experience in 111 patients treated at the European Institute of Oncology in Milan with quadrantectomies and different immediate reconstruction techniques such as glandular flaps, nipple-areola transposition, mastopexy, reduction mastoplasty, round block, implants and musculocutaneous flaps with overall good results in 77.5%, fair in 17% and poor in 5.5% of patients. Although the rate of good results was high, these procedures were very aggressive both from the oncological resection and the reconstruction points of view.
Veronesi’s experience with lumpectomy (25) demonstrated that this procedure is as effective as quadrantectomy with or without skin resection but with fewer sequelae. Subsequently there were publications about the unnecessary wide margins to ensure oncologic safety as shown by the SSO/ASTRO Consensus guideline recommendations (27), where positive margins (invasive or in situ tumor reaching the ink) were associated with a twofold increased risk of local recurrence compared to negative margins but wide margins did not reduce the rate of local events significantly compared to no ink in the tumor, which led to increasingly more limited resections.
In spite of all these changes, sequelae of conservative treatment with varying degrees of complexity continued to occur in approximately 30% of patients (5-18), leading to the development and improvement of different oncoplastic surgery techniques to prevent deformities and asymmetries secondary to surgery and radiotherapy.
The downscaling of procedures in oncology has also reached surgery and the current objective in order to avoid sequela, is to try to use less invasive techniques and obtain optimal results that do not alter and, if possible, improve the quality of life of patients.
Wazer et al. (28) described that palpable tumors (P=0.046), resected gland volume (P=0.027), tumor bed re-excision (P=0.01), number of radiation fields (P=0.03), boost application (P=0.01), and chest wall separation at radiotherapy (P=0.01) are the factors negatively influencing cosmetic outcomes.
In this line of thought, the use of immediate fat transfer in the prevention of sequelae in conservative treatment is theoretically speaking, a unique opportunity to avoid these negative effects with a simple, low morbidity procedure that can avoid changes in breast volume, shape and symmetry and improve skin sequelae secondary to radiotherapy.
Previous experience with deferred repair of conservative treatment defects with fat transfer have shown that the indications are wide (18) and the results very satisfactory. Delay et al. (29) in a series of 880 breasts with fat transfer procedures and 10 years of follow up of breast reconstructions report good and very good results in 90% of the cases of conservative treatment sequelae without diagnostic difficulties in the follow up and without increasing the rate of local recurrences.
Schultz et al. (30) evaluated 44 patients who underwent fat transfer after conservative surgery or after breast reconstruction to correct deformities. Patients reported an improvement in irregularities as well as breast shape, in addition to increased volume and improved consistency of the breast tissue. This technique, as proven by de Blacam et al. (31), has been effective in correcting deformities in the upper medial quadrant of the reconstructed breast.
Immediate lipofilling in the prevention of sequelae of conservative treatment has a very recent history. In 2014, Moltó García et al. (19) described a technique of superficial repair of the lumpectomy site with fat injection between the dermis and the breast in The Japanese Breast Cancer Society with good results free of post-radiotherapy retraction.
In 2015, Biazus et al. (20) from the Hospital de Clínicas de Porto Alegre, Brazil (HCPA) published the first experience with immediate lipofilling in conservative treatment with a different technique, reproducing the experience of Coleman (24) with fat transfer in his first 20 cases but placing the fat in the gland around the tumor resection bed and subcutaneously, doubling with this procedure the resected volume. He reported good results and a low complication rate.
Our working group, based on our long standing experience in lipofilling and breast reconstruction in all areas, started using immediate fat transfer in 2017 in patients with primary indication for conservative treatment and another group of patients with indication for conservative surgery after neoadjuvant treatment.
We modified the surgical technique presented in other publications (19,20). We perform the lumpectomy, evaluate the volume of the tumor and the indemnity of the margins and repair the resection defect by approximating the glandular borders after placing titanium clips. The breast ends up smaller and deformed with significant skin defects in several sectors caused by the traction of Cooper’s ligaments. We start lipofilling, first inside the gland, adjacent to the resection and all around the perimeter to increase the breast volume, then in the retroglandular and intrapectoral region to provide better breast projection and finally subcutaneously in the whole area where the deformity was present. Then we perform Rigottomies with 18 G needles in order to section the Cooper ligaments through the skin, to correct the retractions and allow the fat to spread. We finish with a gentle massage to complete the breast modelling. All these steps are essential to optimize the results.
Biazus et al. (20) reported that this technique is a very good alternative for small or medium sized breasts and for areas with defects that are difficult to solve such as the upper medial quadrants. In larger breasts when it is necessary to resect tumors in any quadrant the first choice is, in general, therapeutic mastoplasty.
In our series, transferring fat into different areas to correct resection defects, preserving or augmenting the size of the breast to maintain symmetry allowed us to transfer larger volumes (range, 45–225 g) with very good results and free of imaging issues that could potentially increase diagnostic doubts in breasts of all sizes.
Injection with the 1.6 mm Khouri cannula allows large volumes of fat to be injected into subcutaneous tunnels that remain viable as a result of the good integration and vascularization of the graft as described by Carpaneda et al. (32).
The fact that 45.6% of the patients in our series had large breasts and very good objective and subjective results (85%) (Figures 11,12), encouraged us to offer immediate lipofilling as an alternative to therapeutic mastoplasty to this type of patients, with the advantage of it being a simpler technique, with faster recovery, few complications and without having to correct the symmetry of the other breast but if necessary, it can be done with grafted fat.
The procedure was proved to be of low complexity, which implied it could be performed as an outpatient procedure in most of the patients. The complications we observed were few (5.49%) and of low magnitude with the exception of a necrosis of the abdominal donor area that was corrected with excision and subsequent dermolipectomy. This is consistent with other publications (20-33).
The evaluation of the cosmetic results was in general good and very good in most of the patients (89%) and this is consistent with other series (19,20) even in “difficult to correct” areas such as the inner quadrants. These good results were not affected in the patients who required a reoperation to widen margins (4.3%). The area that led to lower satisfaction scores was the lower medial quadrant, particularly in medium sized and ptotic breasts. We believe that in this type of patients, the hypotrophic condition of the skin, the low volume of mammary gland and ptosis influenced the poor incorporation of fat and the residual sequelae and deformities.
As in other publications that show subjective and objective cosmetic outcome evaluation scores (20) we found that the independent observer’s rating was generally lower than the patients’ opinion. It is important to highlight the experience of Khan et al. (34) who evaluated patients with immediate lipofilling versus a control group with breast conservation without fat transfer using the Breast QTM questionnaire that showed in a mean follow-up of 36 months, significantly better cosmetic results (P<0.001) and fewer local breast symptoms (P=0.0045) in the lipofilling group.
In an ongoing study, we are comparing our results with quality of life questionnaires to determine if there are coincidences with conventional objective and subjective evaluations. The usual objections to lipofilling for the repair of sequelae of conservative treatment are potential follow up diagnostic difficulties and oncologic safety (34-39).
A deep study was conducted on the potentially negative impact of lipofilling on breast follow up with mammography and ultrasound leading to minimally invasive diagnostic procedures in nodular sequelae or calcifications secondary to fat necrosis. This point led the American Society of Plastic Surgeons (ASPS) to ban lipofilling in 1987 because of its likely interference with early tumor detection (10).
Subsequent studies (11,12) confirmed that breast lipofilling not only did not interfere with diagnosis, rather, that the doubtful BIRADS III and IV images were fewer than with other common plastic surgery techniques such as breast reduction.
Consistent with the literature (20,29,38), our series showed that the diagnostic doubts, categorized as BIRADS III observed in 4 patients, two cases with clustered microcalcifications in mammography and 2 nodules found by ultrasound remained under control and did not evolve. Two other BIRADS IV lesions required needle biopsies and were reported as oily cyst and cytosteatonecrosis.
Since the use of fat transfer for breast reconstruction started being published (14-29), there were many concerns about the likelihood that fat transfer would have a carcinogenic effect. Three questions were raised about whether fat grafting and ADSCs could induce tumor genesis in breast cancer, accelerate the growth of cancer that is not detectable when subclinical, or promote local recurrence of breast cancer (40,41). These points were not clear from basic research work showing cancer cell development in in-vitro tests and animal models after stimulation with the injection of multiple cell types [adipose-derived stromal cells (ASCs)] and biologically active growth factors (42,43).
Petit et al. (16,17) in a multicenter study questioned the oncologic safety of lipotransfer in a subgroup of patients with a history of intraepithelial neoplasms treated with conservative surgery. However, a longer term follow-up by the same authors found that the local recurrence curves were similar to those of the control group without lipotransfer. After several series and publications (44,45) the oncologic safety of lipofilling was confirmed in two meta-analyses. Krastev et al. (46) pooled seven matched cohorts, 12 cohorts and 40 case series with 4,292 patients and Chen et al. (47) included 17 qualified articles with 1,658 patients and came to the same conclusion, that autologous fat transfer did not result in an increased rate of locoregional recurrence in breast cancer patients and that it can be safely used in breast reconstruction.
Although fat transfer has been a controversial topic, earlier studies confirmed there was no association between cancer recurrence and fat transfer for delayed reconstruction. Our study was designed to assess locoregional oncologic events in two biologically distinct scenarios by adding fat transfer immediately in patients with or without prior neoadjuvant therapy.
Biazus et al. (22) in 2018, in a study on 65 patients with conservative treatment and immediate lipofilling reported a local recurrence rate of 0.44% per year in a follow-up of 40.8 months comparable to the local recurrence rate without fat grafting in other series (48). When comparing clinical and pathologic characteristics between the groups with and without recurrence, the only variable significantly associated with cancer recurrence was the presence of lymph node metastases. This finding is in line with other studies showing that axillary lymph node metastasis is the most important predictor of breast cancer recurrence (49,50).
Stumpf et al. (51), in a mean follow-up of 5 years in 65 patients with immediate lipofilling to conservative treatment who were compared to 255 controls without lipofilling in a 1:4 ratio, did not find any significant difference in locoregional recurrence rates between the group with immediate lipotransfer and those who underwent breast-conserving surgery alone. Inclusion of patients with prior neoadjuvant surgery is not reported in this experience.
In our series, with a mean follow-up of 25.21 months (6–47 months) in the group without NEO and 20.90 months (6–48 months) in the group with NEO, none of the patients with or without NEO presented loco-regional recurrences during the study period. Only 2 patients had distant metastases, one without NEO (Luminal B: bone metastases) and one with NEO (triple negative: lung metastases) 2.19%. Both patients had 2 axillary metastatic lymphadenopathies at the definitive pathology diagnosis.
It is possible to assume that eventual recurrences in patients with breast fat grafting are an event related to the natural history of breast cancer, also influenced by tumor characteristics, tumor biology, axillary involvement, variations in preoperative evaluation and the varying quality of conservative surgery and adjuvant or neoadjuvant treatments (52,53).
Conclusions
This technique when properly indicated and performed, is promising in patients with invasive cancer with or without neoadjuvant treatment and indication for conservative treatment particularly when the tumor volume/breast volume ratio is unfavorable to preserve the breast with good results.
It is simple and provides restoration of shape and volume in the resection bed, with a natural texture, but, on the other hand, it demands skilled craftsmanship and the results and possible sequelae or complications are very operator dependent.
Immediate lipofilling proved to be useful in all breast types and sizes and we have only found less satisfactory results in the lower-medial quadrants. The high percentage of good cosmetic results was maintained in all patients beyond 18 months of radiotherapy. This approach can therefore replace more complex procedures, such as therapeutic mastoplasties with simultaneous symmetrization.
Acknowledgments
We thank all the members of the Breast Unit of the Angel H Roffo Institute of Oncology for their collaboration in carrying out this work. This work did not receive any type of financing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nicola Rocco, Giacomo Montagna and Giuseppe Catanuto) for the series “New Perspectives in Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-131/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-131/dss
Peer Review File: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-131/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-131/coif). The series “New Perspectives in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Helsinki Declaration (as revised in 2013). The study was approved by the research and ethics committee of the Mastology Unit of the Roffo Institute (Approval No. 02/16 UM-IAR) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med 1981;305:6-11. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Gray JR, McCormick B, Cox L, et al. Primary breast irradiation in large-breasted or heavy women: analysis of cosmetic outcome. Int J Radiat Oncol Biol Phys 1991;21:347-54. [Crossref] [PubMed]
- Clough KB, Cuminet J, Fitoussi A, et al. Cosmetic sequelae after conservative treatment for breast cancer: classification and results of surgical correction. Ann Plast Surg 1998;41:471-81. [Crossref] [PubMed]
- Paulinelli RR, Ribeiro LFJ, Santos TD, et al. Oncoplastic Mammoplasty with disguised geometric compensation. Surg Oncol 2021;39:101660. [Crossref] [PubMed]
- Rancati A, Gonzalez E, Angrigiani C, et al. Oncoplastic options in breast conservative surgery. Gland Surg 2013;2:163-9. [PubMed]
- Czerny V. Plastischer ersatz der Brustdruse durch ein Lipom. Zentralbl Chir 1895;27:72.
- Bircoll M. Cosmetic breast augmentation utilizing autologous fat and liposuction techniques. Plast Reconstr Surg 1987;79:267-71. [Crossref] [PubMed]
- Report on autologous fat transplantation. ASPRS Ad-Hoc Committee on New Procedures, September 30, 1987. Plast Surg Nurs 1987;7:140-1. [Crossref] [PubMed]
- Brown FE, Sargent SK, Cohen SR, et al. Mammographic changes following reduction mammaplasty. Plast Reconstr Surg 1987;80:691-8. [Crossref] [PubMed]
- Rubin JP, Coon D, Zuley M, et al. Mammographic changes after fat transfer to the breast compared with changes after breast reduction: a blinded study. Plast Reconstr Surg 2012;129:1029-38. [Crossref] [PubMed]
- Coleman SR. Facial recontouring with lipostructure. Clin Plast Surg 1997;24:347-67. [Crossref] [PubMed]
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007;119:1409-22. [Crossref] [PubMed]
- Gutowski KAASPS Fat Graft Task Force. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg 2009;124:272-80. [Crossref] [PubMed]
- Petit JY, Rietjens M, Botteri E, et al. Evaluation of fat grafting safety in patients with intraepithelial neoplasia: a matched-cohort study. Ann Oncol 2013;24:1479-84. [Crossref] [PubMed]
- Petit JY, Maisonneuve P, Rotmensz N, et al. Safety of Lipofilling in Patients with Breast Cancer. Clin Plast Surg 2015;42:339-44. viii. [Crossref] [PubMed]
- Gonzalez E. Delayed Reconstruction after Breast Conserving Surgery. In Oncoplastic and Reconstructive Breast Surgery, edited by Mario Rietjens and Cicero Urban. Chapter 19:177-91. Springer IT; 2013.
- Moltó García R, González Alonso V, Villaverde Doménech ME. Fat grafting in immediate breast reconstruction. Avoiding breast sequelae. Breast Cancer 2016;23:134-40. [Crossref] [PubMed]
- Biazus JV, Falcão CC, Parizotto AC, et al. Immediate Reconstruction with Autologous fat Transfer Following Breast-Conserving Surgery. Breast J 2015;21:268-75. [Crossref] [PubMed]
- Stumpf CC, Biazus JV, Zucatto FSÂE, et al. Immediate reconstruction with autologous fat grafting: influence in breast cancerregional recurrence. Rev Col Bras Cir 2017;44:179-86. [Crossref] [PubMed]
- Biazus JV, Stumpf CC, Melo MP, et al. Breast-Conserving Surgery with Immediate Autologous Fat Grafting Reconstruction: Oncologic Outcomes. Aesthetic Plast Surg 2018;42:1195-201. [Crossref] [PubMed]
- D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS®️ Atlas, Breast Imaging Reporting and Data System. American College of Radiology; 2013.
- Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg 1995;19:421-5. [Crossref] [PubMed]
- Veronesi U, Salvadori B, Luini A, et al. Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur J Cancer 1995;31A:1574-9. [Crossref] [PubMed]
- Petit JY, Garusi C, Greuse M, et al. One hundred and eleven cases of breast conservation treatment with simultaneous reconstruction at the European Institute of Oncology (Milan). Tumori 2002;88:41-7. [PubMed]
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol 2014;32:1507-15. [Crossref] [PubMed]
- Wazer DE, DiPetrillo T, Schmidt-Ullrich R, et al. Factors influencing cosmetic outcome and complication risk after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol 1992;10:356-63. [Crossref] [PubMed]
- Delay E, Garson S, Tousson G, et al. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J 2009;29:360-76. [Crossref] [PubMed]
- Schultz I, Lindegren A, Wickman M. Improved shape and consistency after lipofilling of the breast: patients' evaluation of the outcome. J Plast Surg Hand Surg 2012;46:85-90. [Crossref] [PubMed]
- de Blacam C, Momoh AO, Colakoglu S, et al. Evaluation of clinical outcomes and aesthetic results after autologous fat grafting for contour deformities of the reconstructed breast. Plast Reconstr Surg 2011;128:411e-8e. [Crossref] [PubMed]
- Carpaneda CA, Ribeiro MT. Study of the histologic alterations and viability of the adipose graft in humans. Aesthetic Plast Surg 1993;17:43-7. [Crossref] [PubMed]
- Rietjens M, De Lorenzi F, Rossetto F, et al. Safety of fat grafting in secondary breast reconstruction after cancer. J Plast Reconstr Aesthet Surg 2011;64:477-83. [Crossref] [PubMed]
- Khan LR, Raine CR, Dixon JM. Immediate lipofilling in breast conserving surgery. Eur J Surg Oncol 2017;43:1402-8. [Crossref] [PubMed]
- Motrescu ER, Rio MC. Cancer cells, adipocytes and matrix metalloproteinase 11: a vicious tumor progression cycle. Biol Chem 2008;389:1037-41. [Crossref] [PubMed]
- Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 2007;14:189-206. [Crossref] [PubMed]
- Petit JY, Lohsiriwat V, Clough KB, et al. The oncologic outcome and immediate surgical complications of lipofilling in breast cancer patients: a multicenter study--Milan-Paris-Lyon experience of 646 lipofilling procedures. Plast Reconstr Surg 2011;128:341-6. [Crossref] [PubMed]
- Brenelli F, Rietjens M, De Lorenzi F, et al. Oncological safety of autologous fat grafting after breast conservative treatment: a prospective evaluation. Breast J 2014;20:159-65. [Crossref] [PubMed]
- Carvajal J, Patiño JH. Mammographic findings after breast augmentation with autologous fat injection. Aesthet Surg J 2008;28:153-62. [Crossref] [PubMed]
- Lohsiriwat V, Curigliano G, Rietjens M, et al. Autologous fat transplantation in patients with breast cancer: "silencing" or "fueling" cancer recurrence? Breast 2011;20:351-7. [Crossref] [PubMed]
- Mojallal A, Saint-Cyr M, Garrido I. Autologous fat transfer: controversies and current indications for breast surgery. J Plast Reconstr Aesthet Surg 2009;62:708-10. [Crossref] [PubMed]
- Iyengar P, Espina V, Williams TW, et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest 2005;115:1163-76. [Crossref] [PubMed]
- Martin-Padura I, Gregato G, Marighetti P, et al. The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34+ progenitors able to promote cancer progression. Cancer Res 2012;72:325-34. [Crossref] [PubMed]
- Kronowitz SJ, Mandujano CC, Liu J, et al. Lipofilling of the Breast Does Not Increase the Risk of Recurrence of Breast Cancer: A Matched Controlled Study. Plast Reconstr Surg 2016;137:385-93. [Crossref] [PubMed]
- Claro F Jr, Figueiredo JC, Zampar AG, et al. Applicability and safety of autologous fat for reconstruction of the breast. Br J Surg 2012;99:768-80. [Crossref] [PubMed]
- Krastev TK, Schop SJ, Hommes J, et al. Meta-analysis of the oncological safety of autologous fat transfer after breast cancer. Br J Surg 2018;105:1082-97. [Crossref] [PubMed]
- Chen Y, Li G. Safety and Effectiveness of Autologous Fat Grafting after Breast Radiotherapy: A Systematic Review and Meta-Analysis. Plast Reconstr Surg 2021;147:1-10. [Crossref] [PubMed]
- Botteri E, Bagnardi V, Rotmensz N, et al. Analysis of local and regional recurrences in breast cancer after conservative surgery. Ann Oncol 2010;21:723-8. [Crossref] [PubMed]
- Yoshihara E, Smeets A, Laenen A, et al. Predictors of axillary lymph node metastases in early breast cancer and their applicability in clinical practice. Breast 2013;22:357-61. [Crossref] [PubMed]
- Viale G, Zurrida S, Maiorano E, et al. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer 2005;103:492-500. [Crossref] [PubMed]
- Stumpf CC, Zucatto ÂE, Cavalheiro JAC, et al. Oncologic safety of immediate autologous fat grafting for reconstruction in breast-conserving surgery. Breast Cancer Res Treat 2020;180:301-9. [Crossref] [PubMed]
- Kosasih S, Tayeh S, Mokbel K, et al. Is oncoplastic breast conserving surgery oncologically safe? A meta-analysis of 18,103 patients. Am J Surg 2020;220:385-92. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
Cite this article as: González E, Berman G, Ursino H, Cavallero J, Azar ME, Ipiña M, Mansilla D, Sturla O. Breast-conserving surgery and immediate autologous fat transfer with or without neoadjuvant treatment: indications, technique, cosmetic and oncologic outcomes. Ann Breast Surg 2023;7:24.