
Timing of post mastectomy radiotherapy in immediate or delayed-immediate breast reconstruction: an algorithm to the sentinel first principle
Introduction
About one out of eight women will be dealing with breast cancer throughout their life. The prevalence of this disease has jet-fueled breast cancer research, causing an immense leap in treatment modalities over the last decades. A better understanding of the disease, its subtypes, its genome and its treatment strategies has allowed us to evolve from aggressive to targeted, from debulking to breast conserving and from avoiding death to ensuring quality of life after survival. Despite major advances in medical therapy, surgery remains an indispensable step in breast cancer treatment. The novelties in breast conserving surgery and reconstructive surgery have made the treatment more versatile, which allows the oncoplastic team to provide a tailor-made surgical plan for each patient. The treatment regimen must aim for synergism between the different treatment modalities, without compromising either the oncological or reconstructive objective. While adjuvant therapy may downstage the tumor and make breast conservative surgery possible, adjuvant treatments like radiotherapy might also compromise the reconstructive outcome. Most patients who undergo breast conserving surgery are treated with radiotherapy, whereas the indication for postmastectomy radiotherapy (PMRT) is mainly based on tumor stage and the extent of lymph node involvement. Radiotherapy of the breast is indicated after breast conserving surgery for all invasive tumors, most ductal carcinoma in situ and Paget’s disease. It will also be applied when mastectomy margins were not clear from disease or when the tumors appeared to be more than 4 cm in diameter. Locoregional radiotherapy is indicated when nodal disease is confirmed (≥ N1) and will be more extensive according to the degree of nodal disease.
In patients needing PMRT, the definite reconstruction can be delayed by placing an expander in the mastectomy pocket. Although the consequences of radiotherapy on the autologous reconstructed breast is the subject of discussion, the literature suggests a higher occurrence of fat necrosis, late flap failure and decreased esthetic outcome from radiotherapy after free flap breast reconstruction (1). Since autologous breast reconstruction requires proper organization regarding surgery time and available surgeons, it is not advisable to rely on a preoperative diagnosis to decide whether or not to proceed with an autologous reconstruction.
A problem arises in clinical node-negative breast cancer patients, where the definite tumor and nodal staging is not complete until full tumor and sentinel node resection. It is logistically not feasible to foresee both immediate and delayed reconstructive surgery depending on an intra-operative decision. Therefore, the sentinel first principle was introduced in our center.
This paper outlines the algorithm applied in our center, in which the sentinel procedure is done in a separate surgery before the mastectomy for definite staging. This method, called the “sentinel first procedure”, allows the oncoplastic team to decide whether immediate or delayed-immediate reconstruction is indicated. This paper describes an algorithm for this principle, which was introduced in our center in 2017, and reviews thirteen cases. The objective of this principle is to improve the oncoplastic orchestra of breast reconstructive surgery.
Protocol
Patients with a ductal carcinoma in situ (DCIS) tumor or a tumor that is smaller than 3 cm with a clinically negative node status are candidates for mastectomy with immediate reconstruction if the node is confirmed to be negative after resection. These patients will be included in what is called the “sentinel first” procedure. These patients are scheduled for sentinel node biopsy in the surgical day clinic. When no cancerous cells are found in the resected sentinel nodes, skin sparing mastectomy with immediate autologous or allogenous reconstruction is planned as second surgery. When one or more sentinel nodes are invaded, de patient is scheduled for mastectomy with expander prosthesis reconstruction and the definitive reconstruction will be completed after adjuvant radiotherapy. This is called delayed immediate reconstruction (Figure 1).
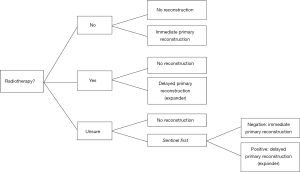
Radiotherapy can be initiated between six weeks and two months after the placement of the expander and when the skin is properly healed. In the case of adjuvant chemotherapy, the initiation of radiotherapy is between two and six weeks after the last chemotherapy session. Completion of the free flap reconstruction can be planned as soon as the skin is sufficiently healed from radiotherapy which varies between 6–12 months.
When autologous reconstruction is not possible or when the patient prefers it, an implant-based reconstruction is second choice. In that case, the timing of radiotherapy will be in a two-stage setting with an expander prosthesis that is similar to autologous reconstruction. Both the expander as the definite prosthesis will be placed in a subpectoral plane. Patients that are initially advised to get a delayed or delayed immediate reconstruction are evidently no candidate for the sentinel first procedure.
Cases
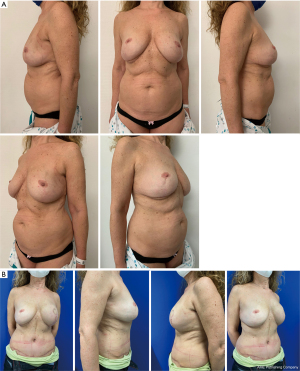
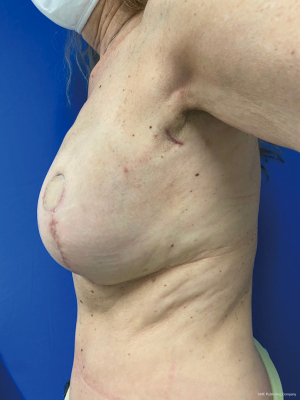
Between 2017 and 2021, a total of thirteen patients were candidates for the sentinel first procedure. Patients were between 45 and 61 years old (mean: 52.1) and they were all diagnosed with either invasive or in situ ductal type carcinoma. The indication for mastectomy was multifocality in four patients, recurrence in three patients, BRCA1 positivity in one patient and unfavorable tumor/breast ratio or esthetic outcome in five patients. Four patients received neoadjuvant chemotherapy for triple negativity which downstaged the tumor to an ypT0N0 stage in three patients. The sentinel was positive (malignant cells found) in two cases, in which an expander was placed to preserve the breast pocket during radiotherapy. The reconstruction was completed as an immediate delayed procedure six months after the completion of the radiotherapy treatment regimen. Nine patients were reconstructed with a DIEaP (Deep Inferior Epigastric artery Perforator) flap, one patient with a PAP (Profunda Artery Perforator) flap, one patient with TMG (Transferse Myocutaneous Gracilis) flap, one patient with implants and one patient with an expander that is not reconstructed yet. Figure 2 shows a 69-year-old patient with history of breast reduction who was diagnosed with multifocal ductal carcinoma in situ in the left breast. A sentinel first procedure was done two weeks after the diagnosis. The sentinel showed no nodal disease, so the patient was planned for a unilateral DIEaP flap reconstruction of the left breast three weeks later. The sentinel procedure leaves a 2 cm scar in the axilla, as seen in Figure 3.
Discussion
Post mastectomy radiotherapy has become an indispensable part of the therapy regimen, since the EBCTCG meta-analysis reported that it improves disease free and breast cancer survival for patients with involved axillary lymph nodes (2). Also, the introduction of the sentinel lymph node biopsy procedure with axillary and thoracic wall radiotherapy has become an alternative to axillary lymph node dissection in patients with limited nodal disease (3,4). PMRT reduces the risk of local recurrence, which most often occurs subcutaneously in the chest wall, followed by the skin itself and mostly at the area around the mastectomy scar. It is postulated that the latter is the result of tumor cell seeding during the surgical procedure (5).
The surgical technique of mastectomy should eliminate as much breast tissue as possible without disrupting surrounding structures that are important for reconstructive outcome. The skin flap should ideally contain only subcutaneous tissue without residual breast tissue, since the skin above the tumor site contains a high risk of recurrence especially in DCIS type breast tumors (6). Patients that are candidates for primary breast reconstruction after mastectomy, the need for adjuvant radiotherapy will determine whether an immediate or delayed immediate reconstruction can be performed. The delayed immediate principle was introduced to decrease the complications associated with radiotherapy on the reconstructed breast, but this also delays reconstruction in patients that turn out to be in no need of radiotherapy. The psychological burden of breast reconstructive surgery is less in immediate procedures compared to immediate delayed. Also, an unnecessary delay of final reconstruction in sentinel negative patients is psychologically challenging for the patient.The need for PMRT cannot be excluded until the final pathological evaluation is done, being after surgery. Although a systematic review from 2014 concluded that there were similar complication rates between patients receiving radiotherapy before and after autologous reconstruction, the overall incidence of flap loss was 1% in patients who received radiotherapy before reconstruction vs. 4% in patients who received radiotherapy after reconstruction (7). A meta-analysis of 12 observational studies concluded the same about complication rates, but stated that women who received radiotherapy after reconstruction had a significantly higher incidence of revisional procedures compared to women receiving radiotherapy before their reconstruction (8). In implant based reconstruction, radiotherapy on the permanent implant is associated with a higher incidence in capsular contracture, but the rate of reconstructive failure is subject to debate. Studies that show an increase in reconstructive failure in patients that receive radiotherapy on their implant are contradicted by a meta-analysis from 2017 that shows no difference between radiotherapy to the tissue expander versus radiotherapy to the permanent implant. Conclusive high-quality evidence from randomized clinical trials is lacking, according to Ho et al. (1). Still, autologous reconstruction is reported to have lower rates of complications and better cosmetic outcomes in the setting of PMRT, compared to implant-based reconstruction (9).
Studies show that there were no differences in complication rate when the autologous reconstruction was performed either before or after six or twelve months post-radiotherapy. Implant reconstruction after prosthesis did show a higher rate of implant failure when the exchange was done within six months of radiotherapy according to a study by Peled et al. (10). In implant-based reconstruction, capsulectomy and Acellular Dermal Matrix can be used to prevent implant failure and improve the surgical outcome.
Conclusions
Immediate postoperative complications might delay the administration of adjuvant therapy administration, while late complications impact the safety of oncological surveillance, cost/effectiveness and aesthetic outcome of the reconstruction. In a certain subpopulation of early stage breast cancer patients in whom tumor size does not require PMRT after mastectomy, the need for PMRT will be almost solely based on the nodal status of the patient. For this purpose, the sentinel first procedure was introduced in our center as a staging tool that allows the surgical team to decide whether immediate or delayed reconstruction should be applied. The long-term outcomes and benefits of this procedure should be determined in larger case series.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tine Engberg Damsgaard and Jørn Bo Thomsen) for the series “Breast Reconstruction—The True Multidisciplinary Approach” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-51/coif). The series “Breast Reconstruction—The True Multidisciplinary Approach” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ho AY, Hu ZI, Mehrara BJ, et al. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol. 2017;18:e742-53. [Crossref] [PubMed]
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [Crossref] [PubMed]
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Sávolt Á, Péley G, Polgár C, et al. Eight-year follow up result of the OTOASOR trial: The Optimal Treatment Of the Axilla - Surgery Or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: A randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol 2017;43:672-9. [Crossref] [PubMed]
- Kaidar-Person O, Poortmans P, Offersen BV, et al. Spatial location of local recurrences after mastectomy: a systematic review. Breast Cancer Res Treat 2020;183:263-73. [Crossref] [PubMed]
- Kaidar-Person O, Offersen BV, Boersma LJ, et al. A multidisciplinary view of mastectomy and breast reconstruction: Understanding the challenges. Breast 2021;56:42-52. [Crossref] [PubMed]
- Kelley BP, Ahmed R, Kidwell KM, et al. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: are current practices ideal? Ann Surg Oncol 2014;21:1732-8. [Crossref] [PubMed]
- Schaverien MV, Macmillan RD, McCulley SJ. Is immediate autologous breast reconstruction with postoperative radiotherapy good practice?: a systematic review of the literature. J Plast Reconstr Aesthet Surg 2013;66:1637-51. [Crossref] [PubMed]
- Cooke AL, Diaz-Abele J, Hayakawa T, et al. Radiation Therapy Versus No Radiation Therapy to the Neo-breast Following Skin-Sparing Mastectomy and Immediate Autologous Free Flap Reconstruction for Breast Cancer: Patient-Reported and Surgical Outcomes at 1 Year-A Mastectomy Reconstruction Outcomes Consortium (MROC) Substudy. Int J Radiat Oncol Biol Phys 2017;99:165-72. [Crossref] [PubMed]
- Peled AW, Foster RD, Esserman LJ, et al. Increasing the time to expander-implant exchange after postmastectomy radiation therapy reduces expander-implant failure. Plast Reconstr Surg 2012;130:503-9. [Crossref] [PubMed]
Cite this article as: Ramaut L, Vanhoeij M, Hamdi M. Timing of post mastectomy radiotherapy in immediate or delayed-immediate breast reconstruction: an algorithm to the sentinel first principle. Ann Breast Surg 2023;7:11.

