
Superparamagnetic iron oxide sentinel lymph node biopsy in early breast cancer: an analysis of artefacts on post-operative breast imaging
Introduction
Sentinel lymph node biopsy (SLNB) is the standard treatment for patients with clinically node-negative breast cancers. The randomized multicentre ALMANAC trial (1) and the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 study (2) have established the role of SLNB in predicting the axillary status in early-stage breast cancer with high technical success rate and accuracy. Radioisotope and blue dye are the most commonly used tracer agents in sentinel lymph node (SLN) mapping and its combined use was recognized as the gold standard with the highest identification rate and lowest false negative rate (1,2). However, both radioisotope and blue dye are associated with drawbacks to the technique such as the administrative requirement in handling and disposal of radioactive substances, potential allergic reactions and permanent skin tattoo.
Superparamagnetic iron oxide (SPIO) is a new mapping agent that is in the spotlight in the recent decade. This magnetic tracer is non-radioactive and can allow more flexibility for tracer injection by surgeons. Multiple clinical studies in Europe and the United States have shown non-inferior nodal detection rate of SPIO to the standard technique of radioisotope with or without blue dye (3-9). Moreover, the detection rate of pathologically positive lymph nodes with SPIO outperform the standard technique in several clinical trials (4-6). We have described in our previous study on the applicability of SPIO as the only localizing agent in SLNB, which had a high success rate of 98.8% (10). This is consistent with the encouraging results from the MONOS trial, where the addition of blue dye did not show further improvement in nodal detection (11). Our centre is currently conducting a prospective randomized controlled trial entitled “Use of superparamagnetic iron oxide (SPIO) versus conventional radioisotope and patent blue dye in sentinel lymph node detection for breast cancer: a randomized controlled trial” for a head-to-head comparison of the two modalities (HKUCTR-2709).
However, SPIO tracer has its own drawbacks too. The most frequent reported in previous studies was the adverse effect of skin discoloration (6-9) and metallic particle residue. The ferromagnetic metal residues accumulating in the surrounding breast tissue after SPIO injection has also been shown to impair the image quality of post-operative breast magnetic resonance imaging (MRI) (12,13). This is particularly problematic in population undergoing high risk breast cancer screening, where annual breast MRI is recommended in the National Comprehensive Cancer Network (NCCN) guidelines (14). There was also a case report on an unexpected finding on post-operative mammography due to accumulation of iron oxide particles (15). The aim of this study is to investigate the effect of SPIO tracer residues on the interpretation of post-operative breast imaging and the correlation with skin discoloration. We present the following article in accordance with the TREND reporting checklist (available at https://abs.amegroups.com/article/view/10.21037/abs-21-110/rc).
Methods
This prospective study was conducted in a University Affiliated Tertiary Breast Cancer Centre in Hong Kong from January 2018 and it is an ongoing study now. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethical Committee of the University of Hong Kong/ Hospital Authority (HKU/HA HKW UW 17-490). Informed written consents were obtained from all patients who were willing to participate.
Patient recruitment and surgery
Patients who were diagnosed with clinical T1–3 invasive ductal or invasive lobular carcinoma and had clinically negative axillary assessment were eligible. Informed written consents were obtained pre-operatively by our specialized breast surgeon and breast care nurse. All recruited patients received breast cancer surgery including SLNB with curative intent in our breast cancer centre. Randomization was performed at 1:1 ratio to either the SPIO arm (study arm) or the conventional arm (control arm). In the study arm, patients received subareolar injection of 2mCi Technectium-99m sulphur colloid in the nuclear medicine department one day before operation. On the day of operation, 2 mL of SPIO tracer (Magtrace, formerly known as SiennaXP) was injected subcutaneously in the subareolar region at least 20 minutes before the start of operation by the operating breast surgeon. SLN localization was performed solely based on the signals from the magnetic tracer. Ex vivo counts of each retrieved SLN were recorded using both the magnetometer and the gamma probe. SLNB was considered complete when the residual count at nodal basin was <10% of the highest ex vivo reading for both the magnetic and radioisotope tracer.
Followup
Post-operatively, patients attended regular followup every 3–6 months in our breast oncology clinic. Routine imaging surveillance with mammography with or without supplementary breast ultrasonography were arranged for cancer surveillance. The extent of skin discoloration was also assessed in each followup. The maximum dimensions of the stained area and intensity of the staining (no, faint or marked staining) were recorded. Nine patients in the SPIO arm who received breast conservative surgery were invited to obtain a followup breast MRI at the Department of Radiology at 18 months. All clinical data was collected and computerized into a database by an independent research assistant.
Breast MRI
All invited patients underwent contrast-enhanced breast MRI with the same MRI scanner, MAGNETOM Aera (Siemens Healthineers, Erlangen, Germany), at a field strength of 1.5 Tesla, using a bilateral breast coil. Our protocol used pre-contrast axial T2W with fat suppression and dynamic contrast axial 3D GR T1W (Bandwidth 340 Hz/pixel, echo time 2.39 msec). Every MRI study was interpreted by two independent breast radiologists (MC, CL) in two separate institutions. Any discrepancy was resolved by mutual discussion and consensus.
The extent of MRI interference was graded according to the categories described by Krischer et al. (13): (I) breast imaging was possible without restriction, (II) image quality was impaired but readable, (III) interpretation was impossible to interpret due to artefacts. The dimensions of MRI void artefacts were measured for each study and any artefact greater than 5 mm was considered of clinical significance.
Mammography and breast ultrasonography
All recruited patients underwent 1 year post-operative routine surveillance with bilateral mammography (Hologic Selenia Dimensions Digital Mammography Unit) and breast ultrasonography (18 MHz; Siemens Acuson S3000 Ultrasound System). Imaging results were reported according to the Breast Imaging-Report and Data System (BIRADS) and managed by specialized breast surgeons accordingly.
Statistical analysis
All patient and tumor characteristics were tabulated by an independent research assistant. Data were summarized as mean (± standard deviation), median (range) or percentage as appropriate. All statistical analyses were performed using IBM SPSS Statistics version 28.
Results
The first nine patients in the SPIO arm of our randomized controlled trial received a followup breast MRI 18 months after the initial operation. The mean age of patients at the time of operation was 58.9 (±12.1) years and their mean body mass index (BMI) was 24.3 (±5.0) kg/m2. All of them were diagnosed with invasive ductal carcinoma, 6 of them with clinical T1 disease and 3 with clinical T2 disease. Seven patients received upfront breast conserving surgery with SLNB and the remaining two underwent operation after neoadjuvant chemotherapy. SLN localization with SPIO was successful in all patients. The median number of SLN harvested was 2 (range, 1–5). Patient #4 had one macrometastatic SLN and underwent immediate completion axillary dissection.
MRI evaluation
Breast MRI was performed in these 9 patients at a median of 552 (range, 545–552) days after the initial SPIO injection and primary operation. Void artefacts were encountered in all recruited patients and all these artefacts were of clinical significance (>5 mm) (see Figures 1-3). The varying degree of breast MRI interference and skin discoloration at post-operative 18 months were demonstrated in Table 1. Image quality was impaired in 4 (44.4%) of the 9 patients, and interpretation was impossible in 2 patients (22.2%) due to the varying extent of artefacts. There was post-operative scar and architectural distortion noted in the axilla of all recruited patients. However, similar void artefacts related to residual ferromagnetic particles were not evident in the axilla.

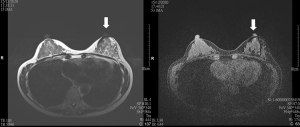
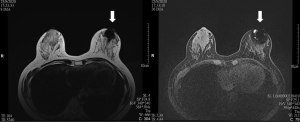
Table 1
| Patient ID | Age | BMI (kg/m2) | Location of tumor from nipple (cm) | Time of MRI since operation (days) | MRI categories [1–3]* | Size of MRI void artefacts^ (mm) | Maximum dimension of skin stain (mm) | Intensity of skin stain (no/faint/marked) |
|---|---|---|---|---|---|---|---|---|
| 1 | 74 | 28.8 | 2 | 552 | 1 | 44×38×5 | 30 | Faint |
| 2 | 44 | 20.2 | 8 | 552 | 3 | 52×46×39 | 0 | No |
| 3 | 69 | 21.6 | 2 | 546 | 1 | 12×30×20 | 10 | Faint |
| 4 | 43 | 19.1 | 5 | 552 | 2 | 41×46×20 | 40 | Marked |
| 5 | 76 | 29.9 | 4 | 545 | 2 | 32×37×30 | 50 | Faint |
| 6 | 56 | 33.2 | 3 | 552 | 1 | 32×32×10 | 50 | Faint |
| 7 | 55 | 22.4 | 6 | 545 | 3 | 44×35×40 | 10 | Faint |
| 8 | 52 | 22.7 | 2 | 552 | 2 | 45×53×41 | 30 | Faint |
| 9 | 61 | 21.0 | 3 | 552 | 2 | 36×35×14 | 50 | Faint |
*, three categories for interpretation of MRI void artefact: 1: breast MRI was possible without restriction, 2: image quality was impaired but readable, 3: interpretation was impossible to interpret due to artefacts; ^, transverse × craniocaudal × anteroposterior (mm). MRI, magnetic resonance imaging; BMI, body mass index.
Mammography and breast ultrasonography evaluation
The first post-operative mammography and breast ultrasonography were performed in these nine patients at a median of 372 (range, 253–841) days. They were graded BIRADS 2 in 7 (77.8%) of the 9 patients and BIRADS 4 in the remaining 2 patients. Patient #1 had a mammographic finding of interval increase in amorphous microcalcifications over the diseased breast and core biopsy was benign. Patient #7 had a newly detected ill-defined density at the periareolar region of the diseased breast, which was away from the site of lumpectomy (see Figure 4). Subsequent core biopsy yielded dark pigmented material from all three samples and pathology showed extensive fibrosis with heavy deposition of haemosiderin pigment granules.
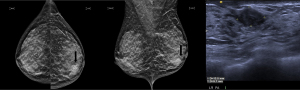
Post-operative skin discoloration
Most patients (88.9%) had residual brownish skin hyperpigmentation at the site of SPIO injection in their post-operative 18 months. The proportion of patients with marked skin stain dropped from 55.6% at post-operative 3 months to 11.1% at post-operative 18 months. The residual skin stain was mostly faint, with a mean maximal dimension of 30 (±19.4) mm at post-operative 18 months. More importantly, the dimension and intensity of skin stain did not correlate well with the degree of MRI interference (see Table 1). Patient #2 was the only one in this cohort to have resolved skin hyperpigmentation. However significant void artefacts were encountered on her post-operative breast MRI. Similarly, patient #7 had minimal residual skin stain of 10 mm only, but her breast MRI was graded impossible to interpret and there were possible artefacts in her mammography and ultrasonography. There were otherwise no adverse events reported among these nine patients.
Discussion
In light of the intrinsic limitation of radioisotope and blue dye, SPIO has challenged the role of the standard technique in SLN mapping in early stage breast cancer. Multiple non-inferiority trials (3-9) and meta-analyses (16,17) have repeatedly validated the high SLN detection rate with SPIO, in particularly in the detection of nodal involvement (4-7). The reported success rate of SLN localization per patient ranged from 94–98% (3-9,16,17), which was comparable with the standard technique of radioisotope and blue dye. The simplified logistic and consistently high concordance rate with the standard technique have encouraged the use of SPIO as a sole tracer in SLN mapping (10,11,18). However, distortion of post-operative breast MRI images was commonly described after SPIO guided SLN mapping (12,13,15) and rarely in mammography. With our study, we have evaluated the effect of SPIO tracer residues on post-operative breast imaging and correlated with the extent of skin discoloration.
MRI artefacts exist in patients who have received breast cancer operation with the conventional tracers (19,20). Seromas, inflammation and fat necrosis could show up as rim enhancing breast lesion in post-operative MRI. The presence of architectural distortion, fibrosis and skin thickening in the post-operative period would also result in the misinterpretation as malignancy or recurrence. The presence of metallic artefact in patients with wide local excision, on the other hand, makes detection of small local recurrence difficult. However, appropriate surgical history, addition of contrast material administration with kinetic assessment and sometimes second look ultrasonography could aid the correct interpretation of post-surgical findings in most circumstances.
Currently, there is no long term data regarding the duration of these SPIO-associated MRI artefacts. A prospective Croatian study (21) evaluated post-operative breast MRI in 9 patients at the time frame of 6 months after surgery, until 12 months after surgery and found artefacts on every performed study. Huizing et al. (12) found the persistence of MRI artefacts in one patient at 25 months after injection. In the Nordic SentiMag trial (16), all patients who had residual skin discoloration were accompanied by residual transcutaneous magnetic activity for up to 515 days after operation. Therefore, in our study, a time frame of 18 months after operation was chosen in the evaluation of breast MRI artefacts and skin discoloration. Only patients who received breast conservative surgery were invited for post-operative breast MRI, as we believed this was the group of patients most affected by the presence of MRI interference. Our results were in accordance with the study by Krischer et al. (13), in which breast MRI was performed at post-operative 42 months. Around 20% of the MRI images were graded impossible to interpret. Karakatsanis et al. (22) suggested a possible correlation between size and intensity of skin discoloration, and the extent of artefacts on MRI. However, in our small prospective series, the extent of skin discoloration did not predict the size and degree of MRI artefacts. To our understanding, there is yet no clear association between these two variables in literature.
In order to address the problem of SPIO-associated MRI incompatibility, Karakatsanis et al. (11) advocated a deeper peritumoral injection of SPIO without compromising the SLN detection rate. The excision of injected tissue during lumpectomy resulted in less residual iron oxide particles, and less skin discoloration. Similar effect on the degree of MRI artefacts is therefore expected. Indeed, this positive effect of excision of injected tissue was indirectly reflected in our current study. It is the usual practice in our institution to inject SPIO at a superficial subareolar region. In the three patients who had minimal post-operative MRI artefacts, the tumors were located close to the periareolar region or a periareolar skin incision was used, where lumpectomy led to most of the tracer being removed. In the remaining six patients, the tumors were more distant from the nipple-areola complex and there were more severe MRI artefacts. Researchers have also investigated on the optimal dosage of SPIO injection (23). Despite a non-inferior nodal detection rate, there was currently no statistically significant difference in the incidence and size of SPIO skin staining with a reduced dosage of injection. The prospective POSTMAG MRI trial (24) is currently under way and hopefully will be able to address the effect of a reduced dose of peritumoral injection of SPIO on SLN detection rate and post-operative breast MRI.
Mammography with or without breast ultrasonography is the more common imaging modality of choice for breast cancer surveillance (25). Abnormal finding associated with SPIO injection was uncommon in post-operative mammography and has only been described in one case report (15). In our ongoing randomized controlled trial, fourteen patients with breast conservative surgery in the study arm have undergone their first post-operative mammography and breast ultrasonography. To date, only one patient encountered SPIO-associated abnormality in her surveillance breast imaging, which resulted in core biopsy. The location of biopsy-proven abnormality was compatible with the site of SPIO injection and was away from the site of lumpectomy. We believe this is a rare but genuine occurrence of SPIO-associated abnormal breast imaging and should raise the awareness of both radiologists and breast surgeons.
The small sample size in this cohort is considered a limitation of this study. However, our results were in line with the best available evidence in literature and it is the first study to correlate the degree of MRI interference and extent of skin discoloration at the same time. Apart from the several means to reduce post-SPIO MRI incompatibility, further studies should also focus on the temporal change in MRI artefacts and evaluate the duration of MRI interference.
Conclusions
In conclusion, SPIO-guided SLNB is associated with inevitable void artefacts with post-operative breast MRI and rarely results in abnormality in mammography and breast ultrasonography. MRI imaging quality was affected in majority of patients at post-operative 18 months. Future research is needed to reduce the extent of MRI interference in order to extend the application of SPIO in breast cancer patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://abs.amegroups.com/article/view/10.21037/abs-21-110/rc
Data Sharing Statement: Available at https://abs.amegroups.com/article/view/10.21037/abs-21-110/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://abs.amegroups.com/article/view/10.21037/abs-21-110/coif). AK serves as an unpaid editorial board member of Annals of Breast Surgery from September 2019 to August 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethical Committee of the University of Hong Kong/ Hospital Authority (HKU/HA HKW UW 17-490). Informed written consents were obtained from all patients who were willing to participate.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goyal A, Newcombe RG, Chhabra A, et al. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer--results of the ALMANAC validation phase. Breast Cancer Res Treat 2006;99:203-8. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 2007;8:881-8. [Crossref] [PubMed]
- Douek M, Klaase J, Monypenny I, et al. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG Multicentre Trial. Ann Surg Oncol 2014;21:1237-45. [Crossref] [PubMed]
- Thill M, Kurylcio A, Welter R, et al. The Central-European SentiMag study: sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast 2014;23:175-9. [Crossref] [PubMed]
- Piñero-Madrona A, Torró-Richart JA, de León-Carrillo JM, et al. Superparamagnetic iron oxide as a tracer for sentinel node biopsy in breast cancer: a comparative non-inferiority study. Eur J Surg Oncol 2015;41:991-7. [Crossref] [PubMed]
- Rubio IT, Diaz-Botero S, Esgueva A, et al. The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. Eur J Surg Oncol 2015;41:46-51. [Crossref] [PubMed]
- Houpeau JL, Chauvet MP, Guillemin F, et al. Sentinel lymph node identification using superparamagnetic iron oxide particles versus radioisotope: The French Sentimag feasibility trial. J Surg Oncol 2016;113:501-7. [Crossref] [PubMed]
- Ghilli M, Carretta E, Di Filippo F, et al. The superparamagnetic iron oxide tracer: a valid alternative in sentinel node biopsy for breast cancer treatment. Eur J Cancer Care (Engl) 2017; [Crossref] [PubMed]
- Alvarado MD, Mittendorf EA, Teshome M, et al. SentimagIC: A non-inferiority trial comparing superparamagnetic iron oxide versus technetium-99m and blue dye in the detection of axillary sentinel nodes in patients with early-stage breast cancer. Ann Surg Oncol 2019;26:3510-6. [Crossref] [PubMed]
- Man V, Wong TT, Co M, et al. Sentinel lymph node biopsy in early breast cancer: magnetic tracer as the only localizing agent. World J Surg 2019;43:1991-6. [Crossref] [PubMed]
- Karakatsanis A, Daskalakis K, Stålberg P, et al. Superparamagnetic iron oxide nanoparticles as the sole method for sentinel node biopsy detection in patients with breast cancer. Br J Surg 2017;104:1675-85. [Crossref] [PubMed]
- Huizing E, Anninga B, Young P, et al. Analysis of void artefacts in post-operative breast MRI due to residual SPIO after magnetic SLNB in SentiMAG Trial participants. Eur J Surg Oncol 2015;41:S18. [Crossref]
- Krischer B, Forte S, Niemann T, et al. Feasibility of breast MRI after sentinel procedure for breast cancer with superparamagnetic tracers. Eur J Surg Oncol 2018;44:74-9. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Breast Cancer Screening and Diagnosis. Version 2.2018. [Accessed 2 February 2021]. Available online: https://www.nccn.org/
- Arslan G, Yılmaz C, Çelik L, et al. Unexpected finding on mammography and MRI due to accumulation of iron oxide particles used for sentinel lymph node detection. Eur J Breast Health 2019;15:200-2. [Crossref] [PubMed]
- Karakatsanis A, Christiansen PM, Fischer L, et al. The Nordic SentiMag trial: a comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res Treat 2016;157:281-94. [Crossref] [PubMed]
- Teshome M, Wei C, Hunt KK, et al. Use of a magnetic tracer for sentinel lymph node detection in early-stage breast cancer patients: a meta-analysis. Ann Surg Oncol 2016;23:1508-14. [Crossref] [PubMed]
- Vural V, Yılmaz OC. The Turkish SentiMAG feasibility trial: preliminary results. Breast Cancer 2020;27:261-5. [Crossref] [PubMed]
- Ojeda-Fournier H, Choe KA, Mahoney MC. Recognizing and interpreting artifacts and pitfalls in MR imaging of the breast. Radiographics 2007;27:S147-64. [Crossref] [PubMed]
- Harvey JA, Hendrick RE, Coll JM, et al. Breast MR imaging artifacts: how to recognize and fix them. Radiographics 2007;27:S131-45. [Crossref] [PubMed]
- Zekan Vučetić M, Ninčević J. Report on a study of presence of void artifacts on postoperative MRI breast scans after superparamagnetic iron oxide sentinel lymph node biopsy. Libri Oncologici: Croatian Journal of Oncology 2016;44:59-61.
- Karakatsanis A, Obondo C, Abdsaleh S, et al. Optimisation of breast MRI compatibility after sentinel node biopsy with paramagnetic tracers. Eur J Surg Oncol 2018;44:731-2. [Crossref] [PubMed]
- Hersi AF, Pistiolis L, Dussan Luberth C, et al. Optimizing dose and timing in magnetic tracer techniques for sentinel lymph node detection in early breast cancers: the prospective multicenter SentiDose trial. Cancers (Basel) 2021;13:693. [Crossref] [PubMed]
- Postoperative breast MRI in patients undergoing sentinel node biopsy using super paramagnetic iron oxide nanoparticles. Available online: https://www.isrctn.com/ISRCTN85167182
- Swinnen J, Keupers M, Soens J, et al. Breast imaging surveillance after curative treatment for primary non-metastasised breast cancer in non-high-risk women: a systematic review. Insights Imaging 2018;9:961-70. [Crossref] [PubMed]
Cite this article as: Man V, Cheung M, Lo C, Lam TPW, Kwong A. Superparamagnetic iron oxide sentinel lymph node biopsy in early breast cancer: an analysis of artefacts on post-operative breast imaging. Ann Breast Surg 2023;7:16.

