
Advances in deep inferior epigastric perforator flap breast reconstruction
Introduction
The deep inferior epigastric perforator (DIEP) flap was initially described by Koshima as a muscle sparing variation of the transversus abdominis myocutaneous (TRAM) flap for reconstruction throughout the body, including head and neck and extremity wounds (1,2). It was soon thereafter described for use in breast reconstruction for patients with breast cancer (3-5). Early works demonstrated DIEP flaps had lower rates of post-operative abdominal wall weakness, equal operative times, and no significant differences in post-operative flap-related complications when compared to traditional muscle inclusive TRAM flaps (6-8). Longitudinal trend analysis has proven it to be the primary choice for breast reconstruction decades later (9). Since its initial description, several notable advances and refinements have been made in the planning, execution and modifications of the DIEP flap that are worth reviewing.
Pre-operative computed tomography angiography
Traditionally, pre-operative planning for DIEP flap perforator dissection included using pencil dopplers to locate and map out perforators on the abdominal wall and guide dissection. More recently, the advancement of computed tomography angiography (CTA) imaging has augmented pre-operative assessment of abdominally based perforator flaps.
CTA is typically performed with a high-speed injection of 80–100 cc of contrast medium. Patients are scanned from the pubic symphysis up to 2–4 cm above the umbilicus. Image slide thickness may vary from 0.6–1 mm, with or without three-dimensional reconstruction (10-12). Total post-processing time between radiology and surgeon for maximum intensity projections and three-dimensional volume-rendered reconstruction takes an average of thirty minutes. Perforators are identified and mapped based on location, size and course (12). Disadvantages of this imaging modality include need for radiation and iodinated contrast material, which may cause some degree of vasospasm and cause inaccurate under-representation of smaller caliber perforators (13).
One strategy for flap dissection planning based on CTA involves using the images to map perforators on a grid based on the patient’s umbilicus. This grid is then transposed onto the patient’s abdomen while supine in the operative room (14). Using a method such as this has also been shown to not only improve on operative and flap dissection times, but also has been shown to decrease operative stress on surgeons (10).
In one prospective study, women undergoing breast reconstruction with abdominally-based free flap were evaluated with pre-operative CTA and dominant perforators were chosen and mapped based on these images. Intra-operatively, perforator presence and location were compared to imaging-based mapping and in all 36 patients, there were no false positives or false negative in perforator location with 100% predictive value of CTA in choosing dominant vessels. In a larger subsequent series by the same group, all perforator flap dissections were guided by pre-operative CTA and 93.7% of patients were found to have 2 or more potential suitable perforators in each hemi-abdomen and perforator flaps based on these chosen vessels were found to have lower rates of partial flap necrosis (15). This finding has since been replicated and held up in larger systematic reviews (16). Use of pre-operative CTA for perforator dissection planning has been reported to decrease operative time by 1-2 hours in unilateral delayed breast reconstruction patients, lower intra-operative blood loss, and lead to shorter hospital length of stay (11,17,18).
CTA has also been showed to be superior to other imaging modalities for pre-operative planning. In an early study evaluating use of pre-operative CTA for perforator mapping, six patients were evaluated with pre-operative imaging and no unreported perforating vessels were found intra-operatively (19). This is in contrast to magnetic resonance angiography, which has been found to miss identifying main perforators in two of ten patients evaluated in one series (20). When compared to other pre-operative mapping and imaging modalities—namely handheld doppler, MRA, color doppler, and digital subtraction angiography—pre-operative CTA was found to be more useful for visualizing details for caliber and course of vessels and more accurate at mapping smaller perforators (13,15).
Pre-operative CTA can be used to help guide operative planning beyond just perforator dissection. In a series by Davis et al., CTA were utilized to predict flaps that would later go on to experience venous congestion. Patients who suffered from post-operative venous congestion were more likely to have had atypical venous connections between deep and superficial systems compared to controls identified on CTA (66.7% vs. 8%, P<0.001), with a positive predictive value of 83%. Authors also founds that flaps that did not go on to have venous congestion exhibited more normal connections and more cranially located perforators (21). CTA imaging has also be shown to be useful in aiding flap design for those patients with prior abdominal surgery pre-existing scars (22). In one study, patients with prior abdominal midline scars and large, ptotic contralateral breasts underwent breast reconstruction with conjoined bipedicle abdominally based flaps. Perforator dissection was planned with pre-operative CTA determining what size and which perforators were dominant, and imaging helped decide if intra-flap anastomoses were to be performed in an anterograde or anterograde/retrograde fashion (23).
For many large centers, CTA imaging is part of the routine pre-operative work-up for DIEP flap reconstruction, and refinements in imaging quality and technology continue to make this a powerful tool for microsurgical planning
Intra-operative fluorescence angiography
In addition to preoperative imaging assessment for flap planning, intra-operative imaging may help with evaluation of flap perfusion in real time. Indocyanine green angiography has been widely utilized in plastic surgery for evaluation of perfusion of perforator flaps and vascular territories (24).
Indocyanine green (ICG) dye is a fluorescent substance with absorption and emission peaks in the near-infrared wavelength range of 800–840 nanometers (25). After intravenous injection of ICG, the substance binds to plasma proteins and remains completely bound. Near infrared range light is poorly absorbed by water or hemoglobin, and when near infrared light is shone on tissues with ICG bound to plasma proteins, water and blood in these tissues appear to fluoresce when visualized with a digital video recorder with an infrared filter. This allows for visualization of blood vessels and areas of perfusion. The half-life of ICG is 3–4 minutes, which allows for repeated examinations (24). ICG is contraindicated for use in patients with an iodine allergy, and while systemic toxicity of ICG is extremely low, there have been some case reports of anaphylactic reaction after use (26,27).
Several commercial systems exist for digital imaging devices (24,28). After images are captured, postprocessing occurs during which comparisons are made between pre-, intra-, and post-injection fluorescence and differences are shown. These differences indicate changes in relative blood flow and perfusion.
Initial works by Holm prospectively evaluated 20 free flaps from all over the body and compared ICG angiography with intraoperative findings. In this series, fluorescence angiography helped the surgeon identify arterial spasm, venous congestion, and regional hypoperfusion in real time (29).
ICG imaging may help identify future areas of nonviability prior being clinically noticeable. In a study performed in DIEP flap rat models, perfusion thresholds were evaluated using ICG angiography. Perfusion of areas directly over perforators was set as 100% and other areas were scaled in comparison to this. ICG angiographic evaluation and concurrent clinical assessments were performed at 0, 24 and 48 hours after free flap anastomosis. Total average flap survival was 84.8%, but areas of flap loss were not clearly demarcated or visible clinically until three days after surgery. Average ICG-evaluated perfusion percentage for viable areas was significantly higher than tissue that was ultimately lost (59.1% vs. 26.8%) and these areas of poor perfusion on ICG angiography intra-operatively correlated directly with those areas of necrosis seen clinically three days after surgery (30). ICG angiography has also been utilized in guiding direct excision of flaps in areas of poor perfusion, and had resulted in guided flap resection in 46% of patients in some series (31-33). Use of intra-operative ICG has repeatedly been found to significantly decrease the odds of having post-operative fat necrosis (31,34-36).
Intra-operative ICG imaging can be particularly beneficial in patient who have undergone prior abdominal surgery as it can be utilized to confirm perfusion of the DIEP flap across the midline in patients who have infraumbilical or suprapubic Pfannelstiel scars (37). ICG angiography has also been showing to improve flap outcomes in patients who have had prior abdominal liposuction. In a study by Casey et al., authors note experience with high rates of partial flap loss and palpable fat necrosis when they performed DIEP flaps in patients with a history of liposuction of the abdomen. In this series 13 patients underwent DIEP reconstruction; half were evaluated for flap resection on clinical grounds alone and half were evaluated with intra-operative fluorescent imaging. Authors found that rates of partial flap loss and fat necrosis dropped from 71.4% to 0% with use of intra-operative ICG angiography (38).
Intra-operative ICG imaging may be performed either prior to flap division at the donor site or after microvascular anastomosis at the recipient. In a study comparing differences by evaluation at both sites found is 83% of cases, ischemic areas identified with ICG angiography were concordant at both donor and recipient site evaluation. In the other 17% of patients, the differences in evaluation measured to be a clinical area of 2 cm2, suggesting differences may not be clinically meaningful (39). A later study interesting found that perform ICG angiography after inset at recipient site resulted in small volumes of flap being resected (35).
Intra-operative ICG angiography is an easy to use tool to guide targeted flap resection in patients with prior abdominal surgery or liposuction and should be utilized in those patients in whom there is doubt about perfusion during DIEP flap reconstruction.
DIEP flap neurotization
In the initial descriptions of DIEP flaps by Allen, flaps were rennervated with neurorrhaphy of a sensory branch of a segmental nerve to the rectus muscle to the fourth intercostal nerve (4). Though not always performed with DIEP flaps for breast reconstruction, neurotization of flaps is becoming more common as techniques and outcomes have continued to improve.
Blondeel et al. evaluated sensory recovery of reconstructed breast with and without nerve coaptation. Several groups were evaluated for sensibility including patient’s native breasts, reconstructed breasts with free TRAM without nerve coaptation, free DIEP without nerve coaptation, and free DIEP with nerve coaptation. In this series, sensory thresholds were measured using a variety of different modalities along with sensory evoked potentials. Results showed that patients who underwent free flap breast reconstruction with nerve coaptation had lower pressure thresholds for detecting sensation than those who did not undergo nerve coaptation. Neurotized reconstructed breasts also had more segments that reacted to cold, warm, vibratory stimuli and had higher rates of return of erogenous sensation compared to non-neurotized reconstructed breasts. Even without nerve coaptation, there was some spontaneous return of sensation but nerve coapted breasts had return of sensation earlier and with higher quality and quantity than non-nerve coapted breasts (40). Subsequent studies have replicated this finding of earlier and improved sensory return with neurotized flaps compared to non-neurotized flaps, both in immediate and delayed reconstruction (41,42).
To perform neurorrhaphy with free flap breast reconstruction, one must identify and preserve the sensory nerves supplying the skin overlying the rectus. The DIEP flap is supplied by mixed segmental nerves that enter the rectus abdominis muscle at variable locations 1cm medial to the lateral border on the anterior surface to two-thirds medial to the lateral border on the posterior surface of the muscle (40). Anatomical cadaver studies have detailed the complex nerve anatomy near the rectus abdominis muscle as nerves travel medially. Branching patterns are variable, and the lower five intercostal nerves continue to give muscular branches to the external and internal obliques as they travel medially. An “intercostal plexus” may form with connections from adjacent intercostal nerves coming back together and forming short trunks. Nerves pierce the lateral rectus sheath and run posteroinferiorly to the muscle for 1–5 centimeters before muscle entry and the point of entry varies. Nerves most commonly enter the muscle as multiple branches (three branches being most common). Motor and sensory branches split, and sensory branches quickly join medial and lateral row perforators forming the neurovascular bundle before running up to the skin (43).
At the recipient site, the 3rd, 4th or 5th intercostal nerves may be used for coaptation. The anterior ramus of the lateral branch of the fourth intercostal nerve is critical for erogenous sensation to the breast and the best candidate for return of this sense. If patients are undergoing immediate reconstruction, the fourth intercostal nerve can be identified during the mastectomy and preserved. If patients are undergoing delayed reconstruction, the lateral branch may be found between two segments of the serratus anterior muscle near the fourth intercostal space at the mid-axillary line and used for coaptation (40). More commonly, the anterior intercostal nerves are used for recipient nerves as their location just superficial to the internal mammary vessels at the site of vascular anastomosis make them easily accessible and identifiable within the operative field (44). Nerves may be brought together and loosely coapted with one to two microsutures with 9-0 or 10-0 nylon in a tension-free manner (40).
Recent studies have compared neurotized flaps with direct coaptation to those with use of a nerve conduit. Direct coaptation was performed with 9-0 nylon suture, whereas in the nerve conduit group a 40-mm hollow tube conduit was utilized and donor and recipient nerves were anchored to opposite ends of the tube with 8-0 nylon. In this series, use of a nerve conduit resulted in significantly lower sensory threshold (improved recovery) compared to direct coaptation (45). Others have advocated for use of nerve allograft to bridge gaps between sensory only donor intercostal nerves to sensory only recipients. Dudic et al. performed a cadaver study of donor and recipient nerves and found that a sufficiently long donor nerve up to 10–12 cm in length could be dissected out, but that this nerve was mixed sensory motor in nature. Use of this donor nerve, they suggest, may contribute to suboptimal sensory recovery as the motor components of the nerve do not contribute to any sensory reinnervation. Instead of this approach, they harvested the sensory only component of the donor intercostal nerve distal to the sensory motor division. This resulted in a shorter donor nerve. To retain flexibility with inset and ensure a tension free coaptation, nerve allograft was utilized to bridge any gaps between donor and recipient (44).
Improving sensory recovery after autologous breast reconstruction has been shown to significantly improve patient’s quality of life and have a significant correlation with objectively measured sensory recovery (46). Continuing to improve in this realm has the potential to greatly impact patient’s sense of wholeness and well-being after surgery.
DIEP flap with vascularized lymph node transfer
For some patients with breast cancer, secondary lymphedema may develop after axillary lymph node dissection, sentinel lymph node biopsy, or chemoradiation therapy. Free flaps from the lower abdomen can be modified to include adjacent lymph nodes and lymphatic vessels to allow for simultaneous vascularized lymph node transplant (VLNT) at the time of microvascular breast reconstruction.
In an early report of this technique, Saaristo describes inclusion of lymph nodes surrounding the superficial circumflex vascular pedicle with the traditional DIEP flap (47). Prior to DIEP-VLNT, pre-operative lymphoscintigraphy may be used to map sentinel lymph nodes in the groin to allow for preservation of these during flap dissection (48). Typically, nodes that can be included in the flap safely without compromising lymphatic drainage of the lower extremity are located between the superficial inferior epigastric vein and the superficial circumflex vein, above the level of the inguinal ligaments and lateral to femoral vessels (48). In this series, the DIEP pedicle was anastomosed to the thoracodorsal vessels. Importantly, Saaristo describes the dissection of the recipient vascular bundle including an extensive scar release and removal of axillary tissue. This has been shown to be a critical component of any lymph node transfer to allow for better drainage from the affected extremity. In this technique, the combined flap was raised on dual vascular pedicles. After DIEP pedicle anastomosis, the tip of the lymphatic portion of the combined adjacent groin flap was evaluated for perfusion. If perfusion was deemed inadequate, then a second arterial anastomosis was performed with the superficial circumflex iliac vessels retrograde into the distal end of the thoracodorsal bundle. The lymphatic tissue was placed into the axilla, covering the axillary plexus and the distal end of this portion of the flap was tunneled to the proximal arm and secured with a single transfixion suture. In this series, extending the DIEP flap in this way increased the operative time by an average of 30 minutes compared to routine DIEP flaps. Patients in this series who underwent combined DIEP-VLNT reported decreases in limb circumferences post-operatively at 3- and 6-month out, had improvements in lymphatic drainage visualized on lymphoscintigraphy, and one-third of patients in this series were able to discontinue use of compression garments altogether (47).
In some early series, patients developed lower abdominal seromas at the lymph node donor site. Since, flap dissection has been modified to leave some fat on the superior abdominal fat on the side ipsilateral to lymph node harvest. This can be used to fill the dead space in the groin and no later seromas were reported after this modification (49).
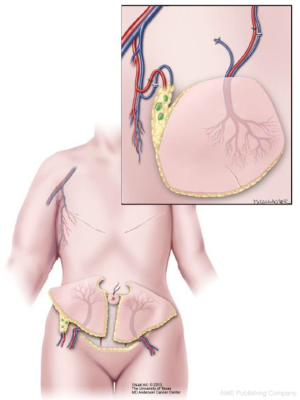
A proposed algorithm for planning DIEP-VLNT flap dissection and inset was reported by Nguyen et al. In addition to lie of the main DIEP vascular pedicle after flap tissue in transferred to the chest, a combined DIEP-VLNT flap must allow for the VLNT to lie in the axilla. This adds an additional challenge to planning the configuration of the extended flap. In the reported series, patients undergoing bilateral reconstruction or those with a history of midline laparotomy incision had their abdominal flap and VNLT designed to both be ipsilateral to the side of reconstruction. In this configuration, the main DIEP pedicle may be anastomosed to the thoracodorsal vessels or the internal mammary artery (IMA) and vein (IMV) if length allows. An additional venous anastomosis of the VNLT pedicle is performed to the axilla. Authors recommend always performing an additional venous anastomosis for the lymph node portion of the flap, but an arterial anastomosis is not routinely necessary (Figure 1) (50).
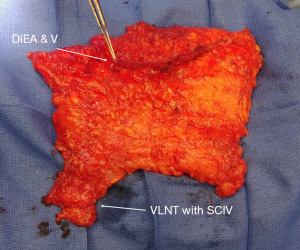
For patients undergoing unilateral reconstruction, an ipsilateral abdominal flap is recommended with the DIEP oriented ipsilateral to the breast to be reconstructed and the VNLT ipsilateral. When transferring the flap to the chest, the tissue must be rotated a full 180 degrees to allow for the main DIEP pedicle to be anastomosed to the IMA/IMV and the VNLT pedicle is anastomosed in the axilla. In this configuration, SCIV is anastomosed to a branch off thoracodorsal vein and arterial anastomosis is not necessary (Figure 2). In this series, 79% of patients had sustained symptomatic improvement after combined DIEP-VLNT and persistent decreased in limb volume up to one year post-operatively (50). A subsequent study demonstrated that this post-surgical reduction in limb volume may be further augmented and seen even earlier in the post-operative period by addition of performing lymph venous bypass concurrent with combined DIEP-VLNT (51).
Patients who undergo combined surgery also report having improvement in quality of life measures, as queried by using LYMQOL. In a series of 18 patients, all 18 had a significant reduction in all domains post-operatively, indicating an improvement in symptoms (49). Additional analysis has shown that combining autologous abdominal free flap with VLNT is a cost effective way of prophylactically preventing the development of post-surgical lymphedema in those patients who have undergone axillary lymph node dissection (52).
Conclusions
Since its initial description, several advances to DIEP flap planning, evaluation, and design have been developed that allow for improved operative execution and post-operative outcomes. As the imaging technology continues to advance, surgeons can gain valuable information about DIEP flap vascularity pre-operatively with CTA and intra-operatively with fluorescence angiography, leading to improved surgical outcomes. Additionally, with the options of neurotizing DIEP flaps and inclusion of VNLT along with abdominal tissue, plastic surgeons can continue to improve patient outcomes and quality of life after breast reconstruction.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Edward I. Chang) for the series “Novel Innovations and Advancements in Breast Reconstruction” published in Annals of Breast Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-87). The series “Novel Innovations and Advancements in Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. DWC serves as an unpaid editorial board member of Annals of Breast Surgery from Mar 2018 to Feb 2022. Both authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Koshima I, Moriguchi T, Soeda S, et al. Free thin paraumbilical perforator-based flaps. Ann Plast Surg 1992;29:12-7. [Crossref] [PubMed]
- Allen JA, Treece P, Dupin CL, et al. Deep inferior epigastric perforator flap for breast reconstruction. Plastic Surgical Forum 1993;31:123-6.
- Allen RJS, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Blondeel PN, Boeckx WD. Refinements in free flap breast reconstruction: the free bilateral deep inferior epigastric perforator flap anastomosed to the internal mammary artery. Br J Plast Surg 1994;47:495-501. [Crossref] [PubMed]
- Blondeel N, Vanderstraeten GG, Monstrey SJ, et al. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br J Plast Surg 1997;50:322-30. [Crossref] [PubMed]
- Craigie JE, Allen RJ, DellaCroce FJ, et al. Autogenous breast reconstruction with the deep inferior epigastric perforator flap. Clin Plast Surg 2003;30:359-69. [Crossref] [PubMed]
- Bajaj AK, Chevray PM, Chang DW. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg 2006;117:737-46; discussion 747-50. [Crossref] [PubMed]
- Healy C, Allen RJ Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg 2014;30:121-5. [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [Crossref] [PubMed]
- Malhotra A, Chhaya N, Nsiah-Sarbeng P, et al. CT-guided deep inferior epigastric perforator (DIEP) flap localization -- better for the patient, the surgeon, and the hospital. Clin Radiol 2013;68:131-8. [Crossref] [PubMed]
- Keys KA, Louie O, Said HK, et al. Clinical utility of CT angiography in DIEP breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:e61-5. [Crossref] [PubMed]
- Smit JM, Klein S, Werker PM. An overview of methods for vascular mapping in the planning of free flaps. J Plast Reconstr Aesthet Surg 2010;63:e674-82. [Crossref] [PubMed]
- Pratt GF, Rozen WM, Chubb D, et al. Preoperative imaging for perforator flaps in reconstructive surgery: a systematic review of the evidence for current techniques. Ann Plast Surg 2012;69:3-9. [Crossref] [PubMed]
- Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [Crossref] [PubMed]
- Teunis T, Heerma van Voss MR, Kon M, et al. CT-angiography prior to DIEP flap breast reconstruction: a systematic review and meta-analysis. Microsurgery 2013;33:496-502. [Crossref] [PubMed]
- Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7. [Crossref] [PubMed]
- Uppal RS, Casaer B, Van Landuyt K, et al. The efficacy of preoperative mapping of perforators in reducing operative times and complications in perforator flap breast reconstruction. J Plast Reconstr Aesthet Surg 2009;62:859-64. [Crossref] [PubMed]
- Alonso-Burgos A, Garcia-Tutor E, Bastarrika G, et al. Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-CT angiography: imaging findings and initial experience. J Plast Reconstr Aesthet Surg 2006;59:585-93. [Crossref] [PubMed]
- Alonso-Burgos A, Garcia-Tutor E, Bastarrika G, et al. Preoperative planning of DIEP and SGAP flaps: preliminary experience with magnetic resonance angiography using 3-tesla equipment and blood-pool contrast medium. J Plast Reconstr Aesthet Surg 2010;63:298-304. [Crossref] [PubMed]
- Davis CR, Jones L, Tillett RL, et al. Predicting venous congestion before DIEP breast reconstruction by identifying atypical venous connections on preoperative CTA imaging. Microsurgery 2019;39:24-31. [Crossref] [PubMed]
- Ngaage LM, Hamed R, Oni G, et al. The Role of CT Angiography in Assessing Deep Inferior Epigastric Perforator Flap Patency in Patients With Pre-existing Abdominal Scars. J Surg Res 2019;235:58-65. [Crossref] [PubMed]
- Cho MJ, Haddock NT, Teotia SS. Clinical Decision Making Using CTA in Conjoined, Bipedicled DIEP and SIEA for Unilateral Breast Reconstruction. J Reconstr Microsurg 2020;36:241-6. [Crossref] [PubMed]
- Liu DZ, Mathes DW, Zenn MR, et al. The application of indocyanine green fluorescence angiography in plastic surgery. J Reconstr Microsurg 2011;27:355-64. [Crossref] [PubMed]
- Still J, Law E, Dawson J, et al. Evaluation of the circulation of reconstructive flaps using laser-induced fluorescence of indocyanine green. Ann Plast Surg 1999;42:266-74. [Crossref] [PubMed]
- Olsen TW, Lim JI, Capone J, A, et al. Anaphylactic shock following indocyanine green angiography. Arch Ophthalmol 1996;114:97. [Crossref] [PubMed]
- Garski TR, Staller BJ, Hepner G, et al. Adverse reactions after administration of indocyanine green. JAMA 1978;240:635. [Crossref] [PubMed]
- Lee BT, Hutteman M, Gioux S, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in perforator flap breast reconstruction. Plast Reconstr Surg 2010;126:1472-81. [Crossref] [PubMed]
- Holm C, Tegeler J, Mayr M, et al. Monitoring free flaps using laser-induced fluorescence of indocyanine green: a preliminary experience. Microsurgery 2002;22:278-87. [Crossref] [PubMed]
- Monahan J, Hwang BH, Kennedy JM, et al. Determination of a perfusion threshold in experimental perforator flap surgery using indocyanine green angiography. Ann Plast Surg 2014;73:602-6. [Crossref] [PubMed]
- Hembd A, Teotia SS, Zhu H, et al. Optimizing Perforator Selection: A Multivariable Analysis of Predictors for Fat Necrosis and Abdominal Morbidity in DIEP Flap Breast Reconstruction. Plast Reconstr Surg 2018;142:583-92. [Crossref] [PubMed]
- Ludolph I, Horch RE, Arkudas A, et al. Enhancing Safety in Reconstructive Microsurgery Using Intraoperative Indocyanine Green Angiography. Front Surg 2019;6:39. [Crossref] [PubMed]
- Pestana IA, Zenn MR. Correlation between abdominal perforator vessels identified with preoperative CT angiography and intraoperative fluorescent angiography in the microsurgical breast reconstruction patient. Ann Plast Surg 2014;72:S144-9. [Crossref] [PubMed]
- Momeni A, Sheckter C. Intraoperative Laser-Assisted Indocyanine Green Imaging Can Reduce the Rate of Fat Necrosis in Microsurgical Breast Reconstruction. Plast Reconstr Surg 2020;145:507e-13e. [Crossref] [PubMed]
- Hembd AS, Yan J, Zhu H, et al. Intraoperative Assessment of DIEP Flap Breast Reconstruction Using Indocyanine Green Angiography: Reduction of Fat Necrosis, Resection Volumes, and Postoperative Surveillance. Plast Reconstr Surg 2020;146:1e-10e. [Crossref] [PubMed]
- Malagón-López P, Vila J, Carrasco-Lopez C, et al. Intraoperative Indocyanine Green Angiography for Fat Necrosis Reduction in the Deep Inferior Epigastric Perforator (DIEP) Flap. Aesthet Surg J 2019;39:NP45-54. [Crossref] [PubMed]
- Louges MA, Bellaiche J, Correia N, et al. Relevance of intraoperative indocyanine green injection in breast reconstruction using DIEP procedure for abdominal scars. Ann Chir Plast Esthet 2016;61:231-4. [Crossref] [PubMed]
- Casey WJ 3rd, Connolly KA, Nanda A, et al. Indocyanine green laser angiography improves deep inferior epigastric perforator flap outcomes following abdominal suction lipectomy. Plast Reconstr Surg 2015;135:491e-7e. [Crossref] [PubMed]
- Malagón-López P, Carrasco-Lopez C, Garcia-Senosiain O, et al. When to assess the DIEP flap perfusion by intraoperative indocyanine green angiography in breast reconstruction? Breast 2019;47:102-8. [Crossref] [PubMed]
- Blondeel PN, Demuynck M, Mete D, et al. Sensory nerve repair in perforator flaps for autologous breast reconstruction: sensational or senseless? Br J Plast Surg 1999;52:37-44. [Crossref] [PubMed]
- Magarakis M, Venkat R, Dellon AL, et al. Pilot study of breast sensation after breast reconstruction: evaluating the effects of radiation therapy and perforator flap neurotization on sensory recovery. Microsurgery 2013;33:421-31. [Crossref] [PubMed]
- Beugels J, Cornelissen AJM, van Kuijk SMJ, et al. Sensory Recovery of the Breast following Innervated and Noninnervated DIEP Flap Breast Reconstruction. Plast Reconstr Surg 2019;144:178e-88e. [Crossref] [PubMed]
- Yap LH, Whiten SC, Forster A, et al. The anatomical and neurophysiological basis of the sensate free TRAM and DIEP flaps. Br J Plast Surg 2002;55:35-45. [Crossref] [PubMed]
- Ducic I, Yoon J, Momeni A, et al. Anatomical Considerations to Optimize Sensory Recovery in Breast Neurotization with Allograft. Plast Reconstr Surg Glob Open 2018;6:e1985 [Crossref] [PubMed]
- Spiegel AJ, Menn ZK, Eldor L, et al. Breast Reinnervation: DIEP Neurotization Using the Third Anterior Intercostal Nerve. Plast Reconstr Surg Glob Open 2013;1:e72 [Crossref] [PubMed]
- Cornelissen AJM, Beugels J, van Kuijk SMJ, et al. Sensation of the autologous reconstructed breast improves quality of life: a pilot study. Breast Cancer Res Treat 2018;167:687-95. [Crossref] [PubMed]
- Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg 2012;255:468-73. [Crossref] [PubMed]
- Chang EI, Masia J, Smith ML. Combining Autologous Breast Reconstruction and Vascularized Lymph Node Transfer. Semin Plast Surg 2018;32:36-41. [Crossref] [PubMed]
- Dancey A, Nassimizadeh A, Nassimizadeh M, et al. A chimeric vascularised groin lymph node flap and DIEP flap for the management of lymphoedema secondary to breast cancer. J Plast Reconstr Aesthet Surg 2013;66:735-7. [Crossref] [PubMed]
- Nguyen AT, Chang EI, Suami H, et al. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann Surg Oncol 2015;22:2919-24. [Crossref] [PubMed]
- Chang EI, Ibrahim A, Liu J, et al. Optimizing Quality of Life for Patients with Breast Cancer-Related Lymphedema: A Prospective Study Combining DIEP Flap Breast Reconstruction and Lymphedema Surgery. Plast Reconstr Surg 2020;145:676e-85e. [Crossref] [PubMed]
- Corkum JP, Bezuhly M. Combining vascularized lymph node transfer with autologous breast reconstruction: A Surveillance, Epidemiology and End Results (SEER) Database cost-utility analysis. J Plast Reconstr Aesthet Surg 2020;73:1879-88. [Crossref] [PubMed]
Cite this article as: Shakir A, Chang DW. Advances in deep inferior epigastric perforator flap breast reconstruction. Ann Breast Surg 2020;4:26.

