Tattoo pigment in axillary lymph nodes mimics occult breast malignancy: a case report
Introduction
Solitary calcified axillary lymph node is a relatively unusual mammographic finding. Its occurrence often raises the suspicion for sinister pathologies such as metastasis secondary to occult breast malignancies or mucin producing non breast tumors. Tattoo pigment is one uncommon benign possibility. Although needle biopsy is widely utilized by clinicians to distinguish benign from malignant breast pathology, surgical excision still remains as a last resort diagnostic option in some situations (1). Tattoo is a common social adornment practice across Asia and in spite of its popularity, there is not yet a case reported in this region. We present the first case in Asia who had complained of severe weight loss and had calcified right axillary lymph node which had mimicked calcification but proven only after excision biopsy to be secondary to tattoo pigment. We present the following article/case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/abs-20-49).
Case presentation
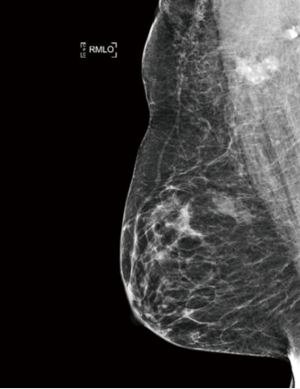
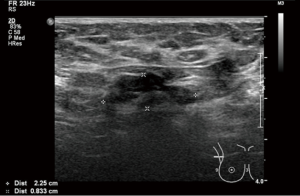
A 45-year-old lady had presented to our clinic with severe weight loss. Her tattoo which had been present for more than 5 years, extended over her right shoulder across to her scapular region. The patient did not report any infective or inflammatory issues, in particular involving the skin. Clinical examination of her right axilla showed the absence of palpable lymph nodes. Bilateral mammography in routine views reported calcified right axillary lymph nodes (Figure 1). Ultrasonography of her right axilla showed borderline enlarged lymph node with preserved fatty hilum and thickened cortex measuring 2.25 cm with calcifications (Figure 2). No other abnormality was seen on either mammography or ultrasound. A computed tomography scan did not show any sign of disease in the thorax, abdomen or pelvis. Core needle biopsy (CNB) of the lymph node under sonographic guidance was performed using a 14 gauge needle. She had no family history of breast cancer or any other cancer. She had no history of autoimmune disorders, medical illness and substance abuse.
Six cores of tissues were obtained and each had a portion of benign lymph node tissue. Scattered amount of melanin laden macrophages with nodular fibrosis were seen with further multiple step sections showed that one of the fibrous nodules had been replaced by amorphous cellular debris. Calcification was present. There was no evidence of malignancy found in any of the sampled tissues.
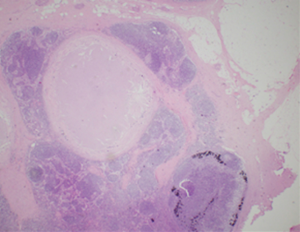
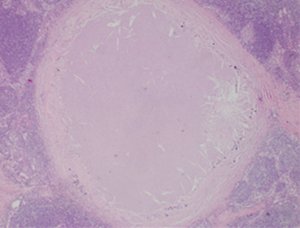
She underwent surgical excision of three suspicious right axillary lymph nodes. Hematoxylin and eosin (H&E) staining was used to prepare all specimen slides. The histological sections revealed well circumscribed nodules having a fibrous wall with central portion comprised of amorphous debris and had showed patchy calcification (Figures 3-5). One of the lymph nodes contained scattered black granules in macrophages (tattoo pigment) which are non-birefringent (Figure 6). No malignancy was seen. Special stain for acid fast bacilli and fungal stain were all negative. This appearance is consistent with reactive lymphadenopathy secondary to tattoo material.
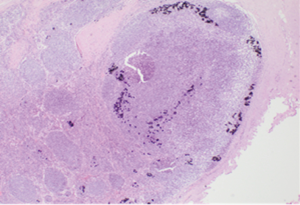
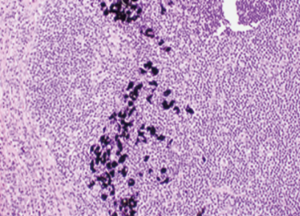
Subsequent follow up mammography did not show any interval suspicious findings and she was discharged to routine screening.
Our institution does not require consent for a case report of one patient.
Discussion
A tattoo refers to the placement of pigment, either intentional or accidental, into the skin (2). The trend of skin tattooing has been popularized in Asia for social cosmetic and medical reasons such as nipple reconstruction following skin preserving mastectomies (3). Tattoo ink comprises of two main components: a dye which is commonly a heavy metal containing compound such as iron oxide, carbon or mercury and a water based or alcohol solvent (4). It has been well established that tattoo pigments can migrate to the regional lymph nodes through the lymphatic vessels after being phagocytosed by the dermal macrophages (5,6). Such pigment can manifest clinically for several years after its application. With medical advancement, tattoo may be removed with laser surgery or dermoabrasion but the nodal pigmentation often persist (7,8). Hence, it is often prudent to make a detailed clinical examination in order to achieve appropriate differential diagnosis.
Distinguishing tattoo pigment from metastases secondary to occult breast malignancies masquerading as intranodal calcification on imaging may be extremely daunting for clinicians (9). Cutting edge imaging modalities can be associated with pitfalls. Tattoo pigment may generate false positive findings on positron emission tomography scan. Iron oxide containing inks have been reported to result in burning or tingling sensation in the skin during magnetic resonance imaging (10,11).
Aside from tattoo pigment, other benign pathologies that resemble similar radiological findings include fat necrosis, gold deposits after chrysotherapy for treatment of rheumatoid arthritis, fat necrosis and granulomatous diseases such as tuberculosis and sarcoidosis (12,13).
Sonographic guided CNB has been vastly accepted as the current gold standard mean of achieving clinical diagnosis with a high diagnostic accuracy rate for breast malignancy and a false negative rate ranging from 0.1% to 3.7% (14-20). Being regarded as a minimally invasive tool, significant complications associated with surgical excision such as wound related issues and seroma can be avoided. Honegger et al. described a case of tattoo pigment leading to nodal calcification which was diagnosed on fine needle aspiration cytology of the axillary lymph node and hence, avoiding the need for CNB (21). Matsika et al. reported the first case of intranodal tattoo pigment in the axillary lymph node where the diagnosis could only be achieved on CNB (22).
Though core needle breast biopsy under imaging guidance is an excellent alternative to surgical excision, sampling error remains a significant factor for false negative results or histologic underestimation. Schueller et al. reported 11 false negative cases of ultrasound guided CNB cases where the biopsy needle had missed the most suspicious area in the sampled lesion (17). In this study, the authors emphasized the importance of the BI-RADS classification for all imaging modalities and the need for procedurists to biopsy the most suspicious region under imaging guidance.
To achieve a reliable histological diagnosis, the number and quality of the sampled specimens obtained are crucial. An adequate sample which is associated with good diagnostic yield should ideally have an uninterrupted length of at least 1 cm and sinks to the bottom of the saline solution (23). Numerous studies have also recommended to sample at least four to five core tissues under ultrasound guidance and suggested that more specimens may be required in lesions with calcifications to reduce false negative rates (24-26). In our case, we yielded a total of 6 core tissue specimens which had sank to the bottom of the specimen bottle following biopsy. At least four specimens were found to have an uninterrupted length of 1cm tissue. However, histological evaluation showed nodal fibrosis which was deemed to be discordant following comparison with the imaging findings. The differential diagnostic possibilities for nodal fibrosis are extremely vast and may range from benign causes such as inflammatory, trauma related, infective to metastatic cancers. Our patient’s presenting symptom was that of severe weight loss and therefore, any form of chronic infection or malignancy cannot be entirely excluded. Hence, our patient was advised for surgical excision.
Magnetic resonance imaging (MRI) has been studied in prior radiological journals as a possible modality to determine malignant infiltration (27-29). Though the nodal size, presence of cortical thickening, irregularity, matting and the absence of fatty hilum in ipsilateral axilla may suggest presence of metastatic nature, there still remains a significant overlap between the MRI features of benign and malignant lymph nodes, thereby limiting its usefulness. The limitation of repeating the CNB in our patient would be that of achieving similar histological result and hence, subjecting our patient to additional cost of biopsy. With careful consideration of the potential options and limitations, surgical excision was offered.
To the best of our knowledge, this is the first case report of tattoo pigment masquerading as occult breast malignancy in Asia and the diagnosis could only be achieved accurately following surgical excision.
Conclusions
Cosmetic tattooing is a common procedure performed across Asia and tattoo pigment should be recognized as a probable cause of axillary nodal changes on mammography that may mimic occult breast malignancy. In spite of the availability of minimally invasive tool such as needle biopsy for diagnostic purpose, excision may still have to be offered as a last resort option to achieve appropriate histological diagnosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/abs-20-49
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs-20-49). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our institution does not require consent for a case report of one patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Walsh R, Kornguth PJ, Soo MS, et al. Axillary lymph nodes: mammographic, pathologic, and clinical correlation. AJR Am J Roentgenol 1997;168:33-8. [Crossref] [PubMed]
- Cruz FA, Lage D, Frigerio RM, et al. Reactions to the different pigments in tattoos: a report of two cases. An Bras Dermatol 2010;85:708-11. [Crossref] [PubMed]
- van der Velden EM, Defranq J, Baruchin AM. Cosmetic and reconstructive medical tattooing. Curr Opin Otolaryngol Head Neck Surg 2005;13:349-53. [Crossref] [PubMed]
- Timko AL, Miller CH, Johnson FB, et al. In vitro chemical analysis of tattoo pigments. Arch Dermatol 2001;137:143-7. [PubMed]
- Sperry K. Tattoos and tattooing. II. Gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 1992;13:7-17. [Crossref] [PubMed]
- Uren RF, Howman-Giles R, Thompson JF. Patterns of lymphatic drainage from the skin in patients with melanoma. J Nucl Med 2003;44:570-82. [PubMed]
- Kluger N, Koljonen V. The surgeon, the tattoo and the black lymph node. J Plast Reconstr Aesthet Surg 2013;66:561-2. [Crossref] [PubMed]
- Manganoni AM, Farisoglio C, Facchetti F, et al. Sentinel lymph node biopsy in melanoma: assessment of risk. Ann Surg Oncol 2008;15:2626. [Crossref] [PubMed]
- Görkem SB, O’Connell AM. Abnormal axillary lymph nodes on negative mammograms: causes other than breast cancer. Diagn Interv Radiol 2012;18:473-9. [PubMed]
- Nam H, Smith S, Laing R. A pitfall of 18-fluorodeoxyglucose-PET in a patient with a tattoo. Lancet Oncol 2007;8:1147-8. [Crossref] [PubMed]
- Offret H, Offret M, Labetoulle M, et al. Permanent cosmetics and magnetic resonance imaging. J Fr Ophtalmol 2009;32:131.e1-3. [PubMed]
- Hooley R, Lee C, Tocino I, et al. Calcifications in axillary lymph nodes caused by fat necrosis. AJR Am J Roentgenol 1996;167:627-8. [Crossref] [PubMed]
- Burdeny DA, Reed MH, Ferguson CA. Calcification of axillary lymph nodes following BCG vaccination. Can Assoc Radiol J 1989;40:92-3. [PubMed]
- Crystal P, Koretz M, Shcharynsky S, et al. Accuracy of sonographically guided 14-gauge core-needle biopsy: results of 715 consecutive breast biopsies with at least two-year follow-up of benign lesions. J Clin Ultrasound 2005;33:47-52. [Crossref] [PubMed]
- Dillon MF, Hill AD, Quinn CM, et al. The accuracy of ultrasound, stereotactic and clinical core biopsies in the diagnosis of breast cancer, with an analysis of false-negative cases. Ann Surg 2005;242:701-7. [Crossref] [PubMed]
- Huang ML, Hess K, Candelaria RP, et al. Comparison of the accuracy of US-guided biopsy of breast masses performed with 14-gauge, 16-gauge and 18-gauge automated cutting needle biopsy devices, and review of the literature. Eur Radiol 2017;27:2928-33. [Crossref] [PubMed]
- Schueller G, Jaromi S, Ponhold L, et al. US-guided 14-gauge core-needle breast biopsy: results of a validation study in 1352 cases. Radiology 2008;248:406-13. [Crossref] [PubMed]
- Youk JH, Kim EK, Kim MJ, et al. Analysis of false negative results after US-guided 14-gauge core needle breast biopsy. Eur Radiol 2010;20:782-9. [Crossref] [PubMed]
- Youk JH, Kim EK, Kim MJ, et al. Sonographically guided 14-gauge core needle biopsy of breast masses: a review of 2,420 cases with long-term follow-up. AJR Am J Roentgenol 2008;190:202-7. [Crossref] [PubMed]
- Zhang C, Lewis DR, Nasute P, et al. The negative predictive value of ultrasound-guided 14-gauge core needle biopsy of breast masses: a validation study of 339 cases. Cancer Imaging 2012;12:488-96. [Crossref] [PubMed]
- Honegger MM, Hesseltine SM, Gross JD, et al. Tattoo pigment mimicking axillary lymph node calcifications on mammography. AJR Am J Roentgenol 2004;183:831-2. [Crossref] [PubMed]
- Matsika A, Bhuvana S, Janet M G, et al. Tattoo pigment in axillary lymph node mimicking calcification of breast cancer. BMJ Case Rep 2013; [Crossref] [PubMed]
- Fishman JE, Milikowski C, Ramsinghani R, et al. US-guided core-needle biopsy of the breast: how many specimens are necessary? Radiology 2003;226:779-82. [Crossref] [PubMed]
- Wu YC, Chen DR, Kuo SJ. Personal experience of ultrasound guided 14-gauge core biopsy of breast tumor. Eur J Surg Oncol 2006;32:715-8. [Crossref] [PubMed]
- Youk JH, Kim EK, Kim MJ, et al. Missed breast cancers at US-guided core needle biopsy: how to reduce them. Radiographics 2007;27:79-94. [Crossref] [PubMed]
- Park VY, Kim EK, Moon HJ, et al. Evaluating imaging-pathology concordance and discordance after ultrasound-guided breast biopsy. Ultrasonography 2018;37:107-20. [Crossref] [PubMed]
- Hyun SJ, Kim EK, Moon HJ, et al. Preoperative axillary lymph node evaluation in breast cancer patients by breast magnetic resonance imaging (MRI): can breast MRI exclude advanced nodal disease? Eur Radiol 2016;26:3865-73. [Crossref] [PubMed]
- Fornasa F, Nesoti MV, Bovo C, et al. Diffusion-weighted magnetic resonance imaging in the characterization of axillary lymph nodes in patients with breast cancer. J Magn Reson Imaging 2012;36:858-64. [Crossref] [PubMed]
- Iima M, Kataoka M, Okumura R, et al. Detection of axillary lymph node metastasis with diffusion-weighted MR imaging. Clin Imaging 2014;38:633-6. [Crossref] [PubMed]
Cite this article as: Lee WP, Shetty SS, Ng VWL, Tan SM. Tattoo pigment in axillary lymph nodes mimics occult breast malignancy: a case report. Ann Breast Surg 2020;4:11.