
Metastatic malignant melanoma: axillary lymphadenopathy and the ugly duckling sign
A 52-year-old woman presented to the breast clinic with a 6-week history of an enlarging and palpable, non-tender left axillary swelling. Fine needle aspiration from the left axillary lymph nodes revealed malignant melanoma; however, the referring team did not identify a primary cutaneous lesion on skin inspection. Screening mammogram and stereotactic biopsy also identified ductal carcinoma in situ of the right breast, treated by wider excision and radiotherapy, and left axillary node clearance identified 2 of 11 nodes as positive for melanoma.
Following referral to oncology and discussion at the local skin cancer multidisciplinary meeting, dermatology reviewed the patient within two weeks. A strikingly atypical 8 mm × 5 mm irregular and nodular melanocytic lesion was excised from her left abdominal wall just below her axillary scar. Histology confirmed a superficial spreading malignant melanoma with Breslow depth 0.6 mm.
There was a delay of 3 months between initial histological nodal diagnosis of melanoma and excision of an obvious cutaneous melanoma close to the ipsilateral axilla. The patient has since had further nodal recurrence requiring completion of the left axillary nodal clearance and treatment with immunotherapy.
What is melanoma?
Melanoma is a relatively rare but aggressive form of skin cancer that arises from proliferation of atypical pigment producing cells called melanocytes. Situated within the basal epidermis, normal melanocytes produce melanin to protect against ultraviolet light (1). They can also be found within the gastrointestinal tract, ears, eyes, oral and genital mucosae and leptomeninges (2). Melanoma may present as a new or changing pigmented skin lesion, but non-pigmented amelanotic melanomas are also seen. Other concerning features of atypical lesions include asymmetry; poorly defined borders; multiple or irregular colours; and diameter over 6mm in size as demonstrated by the lesion in this case (Figure 1).
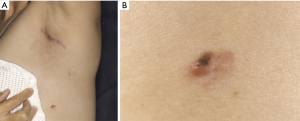
Despite public campaigns to promote sun protection and highlight risk of melanoma, the incidence is rising, particularly in younger adults (3). An increase in recreational sun exposure and sun-bed use have been implicated (1); however, other predisposing risk factors include: paler skin type; family history; age over 50; numerous moles and history of blistering sunburn (4). Staging, surgical and oncological management and ultimately prognosis are determined by ‘Breslow depth’. This is assessed histologically as the depth of invasion of melanocytes from the upper epidermis to the deepest tumour limit (5).
Why is it missed?
Early melanoma can be subtle and difficult to differentiate from benign pigmented lesions. Clinicians with little experience of diagnosing skin cancer are less likely to recognise a new melanoma (1), with further delay in diagnosis potentially occurring with patients who present in childhood when melanoma is relatively uncommon (6); with misdiagnosis of non-pigmented lesions (1) as benign; and in cases of metastatic melanoma from an unknown primary site. Ambiguous histopathology reporting has also been associated with misdiagnosis and therefore all cases must be discussed at skin cancer multidisciplinary meeting (7).
Why does this matter?
Early recognition of melanoma can ensure better prognosis and improved outcomes for patients (1,4). Melanoma can present to any speciality, so it is important that all clinicians, nurses and allied health specialists are vigilant and highlight suspicious skin lesions (7).
How is it diagnosed?
Clinical features
Physicians should remember to apply the ABCD (4) algorithm or the seven-point checklist (4,8) when assessing atypical pigmented lesions that stand out [ugly duckling sign (8)]. Trained individuals can use a polarised light with magnification called a dermatoscope to assist their examination of skin lesion (4).
Investigations
All suspicious lesions must be referred to a dermatologist or member of the skin cancer multidisciplinary meeting for assessment and management. Biopsy should be avoided, with suspicious lesions being initially excised with a 2 mm surgical margin and wider excision at a later stage. The wider surgical margin is dependent on the Breslow depth. Histological indicators of poor prognosis include presence of ulceration; thicker Breslow depth; and large numbers of mitotic figures (4).
Subsequent investigations may include a sentinel node biopsy and/or radiological staging to exclude distant metastasis again depending on the Breslow depth; presence of palpable nodes; or cutaneous metastatic deposits (4).
How is it managed?
All confirmed cases of melanoma are discussed at a specialist skin cancer multidisciplinary meeting (4). Counselling patients about their diagnosis involves a discussion of preventative strategies for further skin cancer. This includes meticulous sun-care protection with high SPF factor and hats; seeking shade particularly during the hottest sun periods between 11 am and 3 pm; performing regular skin checks and highlighting changes to a doctor; and finally, avoidance of sunbeds and sunburn.
It is recommended that baseline vitamin D levels are measured at diagnosis in all patients with melanoma because strict sun avoidance can lead to deficiency (9). Regular follow-up with dermatology is recommended for up to 10 years depending on the stage of the melanoma (4). During these appointments, patients are re-educated and examined for suspicious lesions, lymphadenopathy and hepatosplenomegaly.
Conclusions
We highlight this case, presenting with an obvious ugly duckling lesion and regional lymph node spread, to remind non-dermatology specialists to perform a full skin check when assessing patients with palpable lymphadenopathy. They should refer cases with suspected, or indeed histology proven melanoma, via a red flag or 2-week wait pathway to dermatology or a core member of the skin multidisciplinary meeting for further management.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/abs.2019.01.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Archer CB. Functions of the skin. In: Burns T, Breathnach S, Cox N, et al. editors. Rook’s textbook of Dermatology Volume 1. 9th ed. Oxford: Blackwell Publishing, 2016.
- McCourt C, Dolan O, Gormley G. Malignant melanoma: a pictorial review. Ulster Med J 2014;83:103-10. [PubMed]
- NICE NG14: Melanoma: Assessment and management. National Institute for Health and Care Excellence (2015). Available Online: https://www.nice.org.uk/guidance/ng14 [Accessed 4 December 2018].
- Marsden JR, Newton-Bishop JA, Burrows L, et al. Revised U.K. guidelines for the management of cutaneous melanoma 2010. Br J Dermatol 2010;163:238-56. [Crossref] [PubMed]
- Roberts DL, Anstey AV, Barlow RJ, et al. Guidelines for the management of cutaneous melanoma. Br J Dermatol 2002;146:7-17. [Crossref] [PubMed]
- Dean PH, Bucevska M, Strahlendorf C, et al. Pediatric Melanoma: A 35-year Population-based Review. Plast Reconstr Surg Glob Open 2017;5:e1252 [Crossref] [PubMed]
- Troxel DB. Pitfalls in the diagnosis of malignant melanoma: findings of a risk management panel study. Am J Surg Pathol 2003;27:1278-83. [Crossref] [PubMed]
- NICE Clinical Knowledge Summaries: Melanoma and pigmented lesions. National Institute for Health and Care Excellence (2017). Available online: https://cks.nice.org.uk/melanoma-and-pigmented-lesions [Accessed 4 December 2018].
- Macbeth F, Newton-Bishop J, O'Connell S, et al. Melanoma: summary of NICE guidelines. BMJ 2015;351:h3708. [Crossref] [PubMed]
Cite this article as: Carson C, Murphy B, Kerr O. Metastatic malignant melanoma: axillary lymphadenopathy and the ugly duckling sign. Ann Breast Surg 2019;3:3.

